Assoc. Prof. Zahari P. Vinarov, Ph.D. Chemistry, Ph.D Pharmacy
Head of the Department
Interests
- Oral formulation development and biopharmaceutical characterization
- Intestinal absorption mechanisms
- Amorphous solid dispersions and lipid formulations
- In vitro modelling of the gastrointestinal tract
- Physicochemical aspects of formulation and biopharmaceutics
Bio
Publications
Featured publications
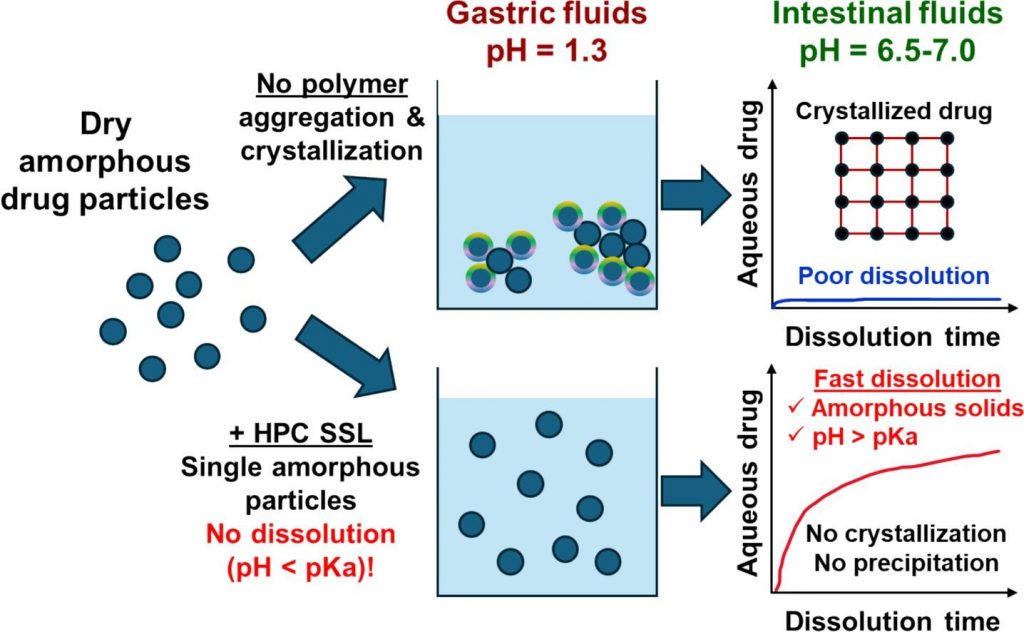
Mechanisms of dissolution and crystallization of amorphous glibenclamide
Amorphous solid dispersions enhance the dissolution and oral bioavailability of poorly water-soluble drugs. However, the link between polymer properties and formulation performance has not been fully clarified yet. We studied the effect of hydroxypropyl cellulose (HPC) polymers molecular weight (Mw) on the storage stability, dissolution kinetics and supersaturation stability of spray-dried amorphous glibenclamide (GLB) formulations. The solid-state stability of amorphous GLB during storage was significantly enhanced by both the 40 kDa (HPC-SSL) and 84 kDa (HPC-L) polymers, regardless of Mw differences. In contrast, HPC-SSL maintained significantly higher aqueous drug concentrations during dissolution, compared to HPC-L (its higher Mw analogue). Dedicated dissolution experiments, in situ optical microscopy and solid-state characterization revealed that aqueous drug concentrations were determined by the interplay between crystallization inhibition, drug ionization, wetting and solubilization effects: (1) HPC prevents surface nucleation, hence inhibiting crystallization, (2) intestinal colloids (bile salts and phospholipids) increase supersaturated drug concentrations via wetting and solubilization effects and (3) pH and drug ionization severely impact the degree of supersaturation. The better performance of the lower Mw HPC-SSL was due to its superior inhibition of surface crystallization during dissolution. These insights into the molecular mechanisms of dissolution and crystallization of amorphous solids provide foundation for rational formulation development.
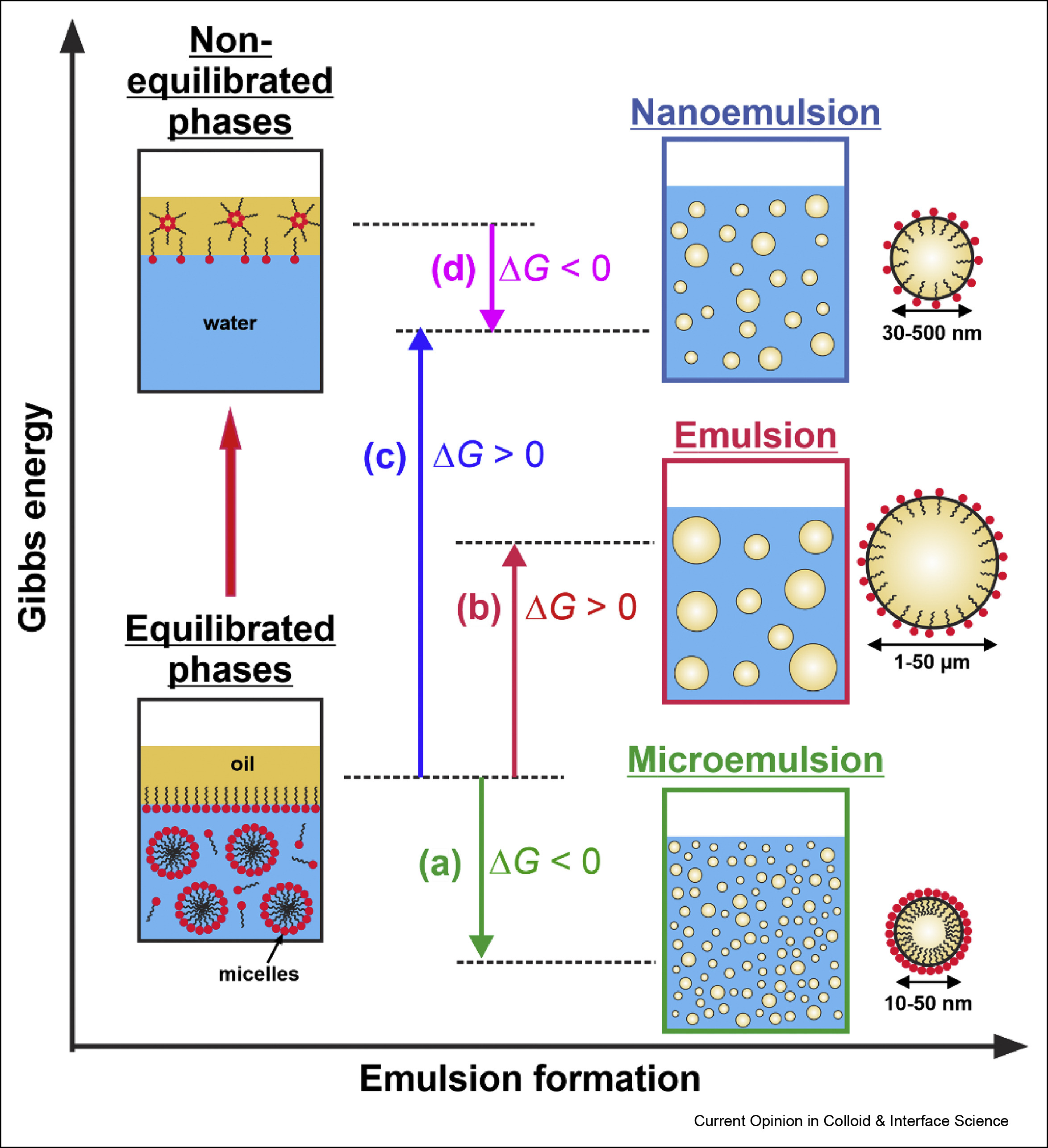
Self-emulsification in chemical and pharmaceutical technologies
The interest in the low energy self-emulsification techniques has exploded in the recent years, driven by three main trends: by the transition to “greener” technologies in both its aspects—less energy consumption and replacement of the petrochemicals by natural ingredients; by the costly and maintenance demanding equipment for nanoemulsification; and by the quest for efficient and robust self-emulsifying formulations for oral drug delivery. Here, we first present a brief overview of the main known low-energy methods for nanoemulsion formation, focusing on their mechanistic understanding and discussing some recent advances in their development and applications. Next, we review three conceptually new approaches for self-emulsification in chemical technologies, discovered in the last several years. The colloidal features and the specific requirements of the self-emulsifying drug-delivery systems (SEDDS) are also discussed briefly. Finally, we summarize the current trends and the main challenges in this vivid research area.
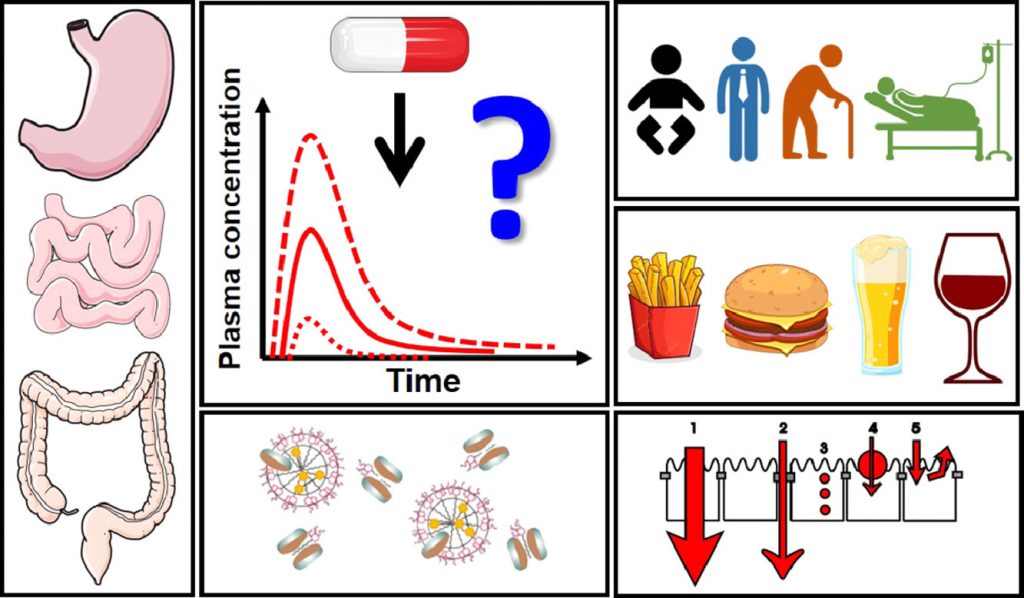
Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review
The absorption of oral drugs is frequently plagued by significant variability with potentially serious therapeutic consequences. The source of variability can be traced back to interindividual variability in physiology, differences in special populations (age- and disease-dependent), drug and formulation properties, or food-drug interactions. Clinical evidence for the impact of some of these factors on drug pharmacokinetic variability is mounting: e.g. gastric pH and emptying time, small intestinal fluid properties, differences in pediatrics and the elderly, and surgical changes in gastrointestinal anatomy. However, the link of colonic factors variability (transit time, fluid composition, microbiome), sex differences (male vs. female) and gut-related diseases (chronic constipation, anorexia and cachexia) to drug absorption variability has not been firmly established yet. At the same time, a way to decrease oral drug pharmacokinetic variability is provided by the pharmaceutical industry: clinical evidence suggests that formulation approaches employed during drug development can decrease the variability in oral exposure. This review outlines the main drivers of oral drug exposure variability and potential approaches to overcome them, while highlighting existing knowledge gaps and guiding future studies in this area.
Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network
Although oral drug delivery is the preferred administration route and has been used for centuries, modern drug discovery and development pipelines challenge conventional formulation approaches and highlight the insufficient mechanistic understanding of processes critical to oral drug absorption. This review presents the opinion of UNGAP scientists on four key themes across the oral absorption landscape: (1) specific patient populations, (2) regional differences in the gastrointestinal tract, (3) advanced formulations and (4) food-drug interactions. The differences of oral absorption in pediatric and geriatric populations, the specific issues in colonic absorption, the formulation approaches for poorly water-soluble (small molecules) and poorly permeable (peptides, RNA etc.) drugs, as well as the vast realm of food effects, are some of the topics discussed in detail. The identified controversies and gaps in the current understanding of gastrointestinal absorption-related processes are used to create a roadmap for the future of oral drug absorption research.

Mechanisms of drug solubilization by polar lipids in biorelevant media
Despite the widespread use of lipid excipients in both academic research and oral formulation development, rational selection guidelines are still missing. In the current study, we aimed to establish a link between the molecular structure of commonly used polar lipids and drug solubilization in biorelevant media. The solubilization of fenofibrate by 13 phospholipids, 11 fatty acids and 2 monoglycerides was studied by an in vitro model of the upper GI tract. The main trends were verified with progesterone and danazol. It was revealed that to alter drug solubilization in biorelevant media, the polar lipids must form mixed colloidal aggregates with the bile. Such aggregates are formed when: (1) the polar lipid is used at a sufficiently high concentration (relative to its mixed critical micellar concentration) and (2) its hydrophobic chain has a melting temperature (Tm) < 37 °C. When these two conditions are met, the increased polar lipid chain length increases the drug solubilization capacity. Hence, long chain (C18) unsaturated polar lipids show best drug solubilization, due to the combination of long chain length and low Tm. Polar lipids with Tm significantly higher than 37 °C (e.g. C16 and C18 saturated compounds) do not impact drug solubilization in biorelevant media, due to limited association in mixed colloidal aggregates. The hydrophilic head group also has a dramatic impact on the drug solubilization enhancement, with polar lipids performance decreasing in the order [choline phospholipids] > [monoglycerides] > [fatty acids]. As both the acyl chain and head group types are structural features of the polar lipids, and not of the solubilized drugs, the described trends in drug solubilization should hold true for a variety of hydrophobic molecules.
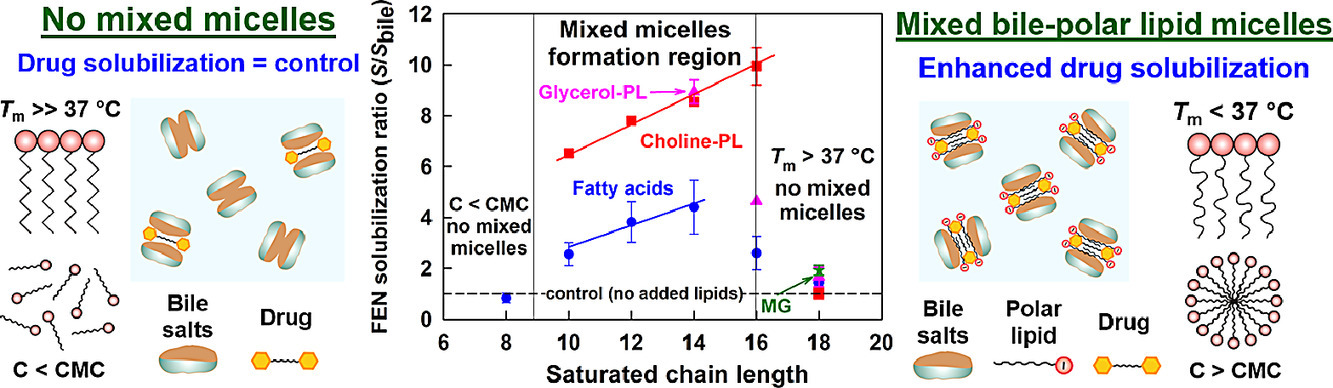
Effect of surfactant-bile interactions on the solubility of hydrophobic drugs in biorelevant dissolution media
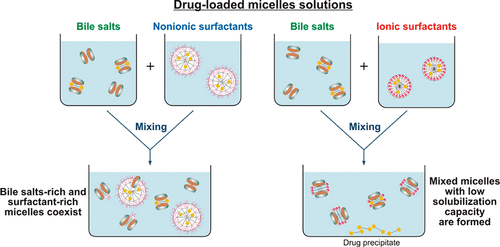
Biorelevant dissolution media (BDM) methods are commonly employed to investigate the oral absorption of poorly water-soluble drugs. Despite the significant progress in this area, the effect of commonly employed pharmaceutical excipients, such as surfactants, on the solubility of drugs in BDM has not been characterized in detail. The aim of this study is to clarify the impact of surfactant-bile interactions on drug solubility by using a set of 12 surfactants, 3 model hydrophobic drugs (fenofibrate, danazol, and progesterone) and two types of BDM (porcine bile extract and sodium taurodeoxycholate). Drug precipitation and sharp nonlinear decrease in the solubility of all studied drugs is observed when drug-loaded ionic surfactant micelles are introduced in solutions of both BDM, whereas the drugs remain solubilized in the mixtures of nonionic polysorbate surfactants + BDM. One-dimensional and diffusion-ordered 1 H NMR spectroscopy show that mixed bile salt + surfactant micelles with low drug solubilization capacity are formed for the ionic surfactants. On the other hand, separate surfactant-rich and bile salt-rich micelles coexist in the nonionic polysorbate surfactant + bile salt mixtures, explaining the better drug solubility in these systems. The nonionic alcohol ethoxylate surfactants show intermediate behavior. The large dependence of the drug solubility on surfactant-bile interactions (in which the drug molecules do not play a major role per se) highlights how the complex interplay between excipients and bile salts can significantly change one of the key parameters which governs the oral absorption of poorly water-soluble drugs, viz. the drug solubility in the intestinal fluids.
Most recent publications
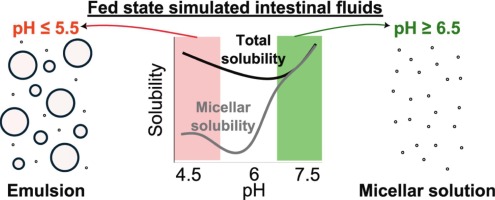
Indirect effects of pH on drug solubility in fed state simulated intestinal fluids
The pH-mediated effect of drug ionization on solubility is well-described. However, pH can also indirectly influence solubility by altering the colloidal structures in human intestinal fluids. This study investigates the indirect pH effect on the apparent solubility of 13 uncharged drugs across a pH range of 4.5 to 7.5 in fed-state simulated intestinal fluids (SIF) composed of taurocholate and lecithin, with or without added lipids (monoolein and/or sodium oleate). A pronounced indirect pH effect on drug solubility was observed when oleate was present in the SIF, whereas monoolein had only a minor effect. Below pH 6.5, sodium oleate was converted to oleic acid, resulting in lipid droplet formation that enhanced lipophilic compound solubility in the total sample (lipid phase + micellar phase), while the micellar solubility remained similar to the reference SIF (without oleate). This resulted in an up to 50-fold increase of the ratio total/micellar drug solubility, which correlated well with drug lipophilicity or its combination with total polar surface area (R2 ≈ 0.8). At higher pH, a lipid phase was not formed because the ionized sodium oleate partitioned in the micellar phase, where it significantly increased drug solubilization. These findings highlight the importance of considering indirect pH effects in solubility assessments by tuning simulated intestinal fluids composition to better reflect in vivo reality.
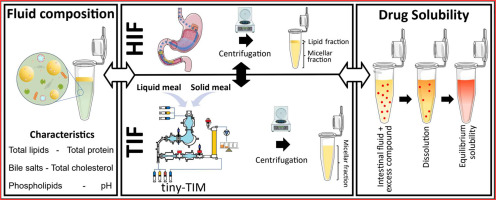
Characterization of human intestinal fluids after the administration of a solid meal
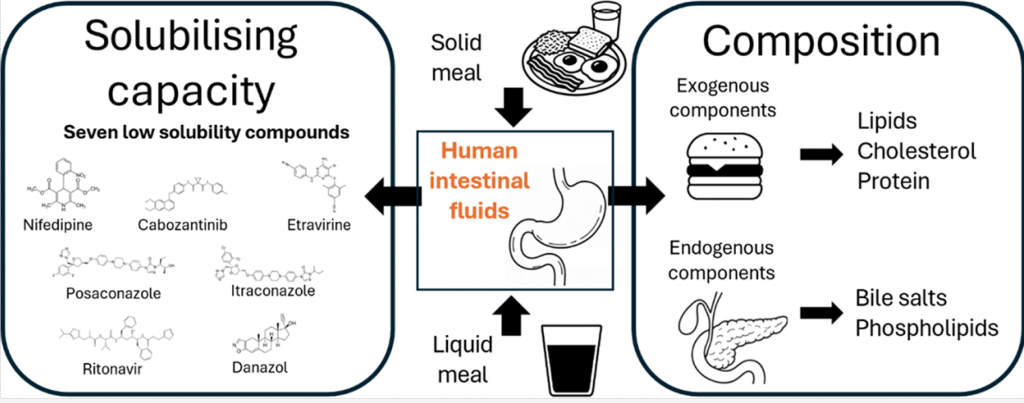
Most available data on the composition and solubilizing properties of postprandial human intestinal fluid (HIF) are derived from studies involving liquid meals. These data inform the development of simulated intestinal fluids, widely used in in vitro assays for predicting intestinal drug behavior. However, the typical human diet primarily consists of solid meals, and the physical form of food has been shown to influence gastrointestinal transit and digestion, thereby affecting drug disposition and bioavailability. This study compares the characteristics of fed-state HIF collected after solid meal ingestion (SM-HIF) with previously published data on pooled liquid meal-derived HIF (LM-HIF) and newly generated data from individual LM-HIF samples. Time-dependent samples were analyzed over 180- and 90 min postprandial sampling periods to assess compositional changes following the administration of a solid and liquid meal, respectively. In addition, pooled samples were used to evaluate the solubilizing capacity for seven lipophilic model compounds. After intake of the solid meal, duodenal concentrations of exogenous (lipids, cholesterol, proteins) and endogenous (bile salts, phospholipids) components gradually increased to peak levels reached after 45−75 min. After 180 min, lipid and protein concentrations were still elevated compared to fasted state levels. In comparison to the liquid meal, the ingestion of the solid meal resulted in reduced concentrations of exogenous components, while endogenous components (bile salts and phospholipids) were relatively similar. For most compounds, the reduction in lipid content led to diminished solubilizing capacity of SM-HIF compared to LM-HIF when considering the combined micellar and lipid fractions. In contrast, the solubilizing capacity of the micellar fraction as such was largely independent of the meal type. Both the composition (particularly the micellar lipid concentration) and the solubilizing capacity of SM-HIF were highly variable between pools, albeit to a lesser extent than in LMHIF. The findings of this study highlight that the physical form of the meal influences the composition and solubilizing capacity of HIF. These insights should be taken into account when refining biorelevant media for in vitro models to better predict food effects during drug product development.
Tiny-TIM intestinal fluids versus human intestinal fluids: A comparison of their composition and solubilizing capacity for poorly soluble drugs
The tiny-TIM system offers an in vitro platform for the simulation of physiological processes occurring in human stomach and small intestine aiding in drug product development by predicting the bioperformance of oral formulations under fasted and fed state intake conditions. To assess this in vitro system in terms of its physiological relevance, we performed a detailed analysis of the composition as well as the solubilizing capacity of tiny-TIM intestinal fluids (TIF), and compared this to previously collected and analysed human intestinal fluids (HIF). Moreover, the impact of meal type on TIF composition and solubilising capacity was investigated by using either a liquid meal or a solid meal. In the fasted state, TIF exhibited lower lipid concentrations with a TIF/HIF ratio of 0.27, and elevated bile salt levels (TIF/HIF ratio of 1.8). Fasted state TIF generally overpredicted the solubilizing capacity of HIF, likely due to its higher bile salt concentrations. In the fed state, TIF contained biorelevant lipid concentrations but remained monophasic without phase separation, unlike HIF. This was likely due to higher bile salt levels (5.3 times that of HIF), which solubilized all lipids into the micellar phase. This resulted on average in a 3.3-fold increase in solubility of the poorly water-soluble model compounds in the micellar fraction of TIF as compared to HIF. Shifting from a liquid to a solid meal had minimal impact on TIF composition and solubilizing capacity.
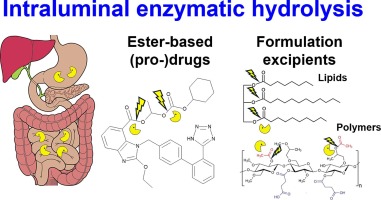
Intraluminal enzymatic hydrolysis of API and lipid or polymeric excipients
The role of intraluminal enzymes for the hydrolysis of active pharmaceutical ingredients (API), prodrugs and pharmaceutical excipients will be reviewed. Carboxylesterases may hydrolyze ester-based API, prodrugs and ester-bond containing polymer excipients, whereas lipases digest lipid formulation excipients, such as mono-, di- and triglycerides. To clarify the conditions that should be mimicked when designing in vitro studies, we briefly review the upper gastrointestinal physiology and provide new data on the inter-individual variability of enzyme activities in human intestinal fluids. Afterwards, the methodology for studying enzymatic hydrolysis of API, prodrugs, lipid and polymeric excipients, as well as the main results that have been obtained, are summarized. In vitro digestion models used to characterize lipid formulations are well described, but data about the hydrolysis of lipid excipients (including surfactants) has been scarce and contradictory. Data on API and prodrug hydrolysis by esterases is available; however, inconsistent use of enzyme types and concentrations limits structure-stability relationships. Hydrolysis of polymer excipients in the lumen has not been significantly explored, with only qualitative data available for cellulose derivates, polyesters, starches, etc. Harmonization of the methodology is required in order to curate larger enzymatic hydrolysis datasets, which will enable mechanistic understanding and theoretical prediction.
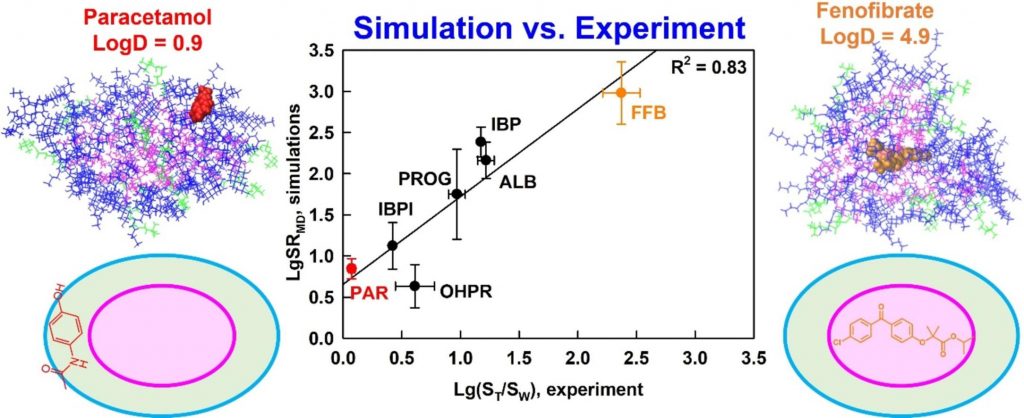
Understanding drug solubilization in intestinal mixed micelles through molecular dynamics simulations
Hypothesis
Solubilization is a fundamental process that underpins various technologies in the pharmaceutical and chemical industry. However, knowledge of the location, orientation and interactions of solubilized molecules in the micelles is still limited. We expect all-atom molecular dynamics simulations to improve the molecular-level understanding of solubilization and to enable its in silico prediction.
Methods
The solubilization of six drugs in intestinal mixed micelles composed of taurocholate and dioleoyl phosphatidylcholine was simulated by molecular dynamics in explicit water and measured experimentally by liquid chromatography. The location and orientation of the solubilized drugs were visualized by cumulative radial distribution functions and interactions were characterized by radial distribution function ratios and hydrogen bonding.
Findings
A new simulation-derived parameter was defined, which accounts for drug-micelle and drug-water interactions and correlates (R2 = 0.83) with the experimentally measured solubilization. Lipophilicity was found to govern the location of all drugs in the micelle (hydrophobic core, palisade layer or on the surface), while hydrogen bonding was crucial for orientation and solubilization of two of the molecules. The study demonstrates that explicit, hydrogen bond-forming water molecules are vital for accurate prediction of solubilization and provides a comprehensive framework for quantitative studies of drug location and orientation within the micelles.