Vasil Georgiev, Ph.D. Candidate
Interests
- Foams and antifoams
- Equilibrium and dynamic surface tension
- Stability of thin liquid films
Publications
Most recent publications
Cosmetic potential of haberlea rhodopensis extracts and extracellular vesicles in human fibroblast cells
Skin ageing is a complex biological process influenced by cellular senescence, oxidative stress, and extracellular matrix degradation. Emerging evidence suggests that plant-derived bioactive compounds and extracellular vesicles (EVs) play a crucial role in modulating cellular homeostasis, promoting tissue regeneration, and counteracting age-related morphological and functional changes. This study investigates the impact of Haberlea rhodopensis in vitro culture extracts, native and enriched with EVs, on key cellular processes, including morphology, mitochondrial dynamics, lysosomal activity, gene expression, and genotoxicity in human dermal fibroblasts. The extracellular vesicles were identified in terms of shape, size, and morphology using dynamic light scattering, negative staining and observation under a transmission electron microscope. A comprehensive in vitro analysis was conducted utilizing light microscopy to assess cellular morphology and lysosomal mass, fluorescence microscopy for actin cytoskeletal organization, mitochondrial integrity, and nuclear morphology, and gene expression profiling for markers associated with collagen synthesis (COL1A1, COL3A1), senescence (CDKN1A), and oxidative stress response (NFE2L2). Additionally, cell cycle progression was evaluated, and genotoxicity was assessed using the neutral comet assay. Haberlea rhodopensis in vitro culture extracts and EVs were found to preserve fibroblast morphology, enhance mitochondrial mass, and upregulate collagen-related gene expression. These effects were concentration-dependent. The extracts exhibited biocompatibility with minimal genotoxic effects, indicating their potential as safe bioactive agents for skin rejuvenation. The findings suggest that Haberlea rhodopensis in vitro culture extracts and their enrichment with extracellular vesicles hold promise for cosmetic and dermatological applications, particularly in enhancing collagen production, preserving cellular integrity, and mitigating age-related alterations in skin fibroblasts. Further studies are warranted to elucidate the underlying molecular mechanisms and optimize formulation strategies for clinical translation.
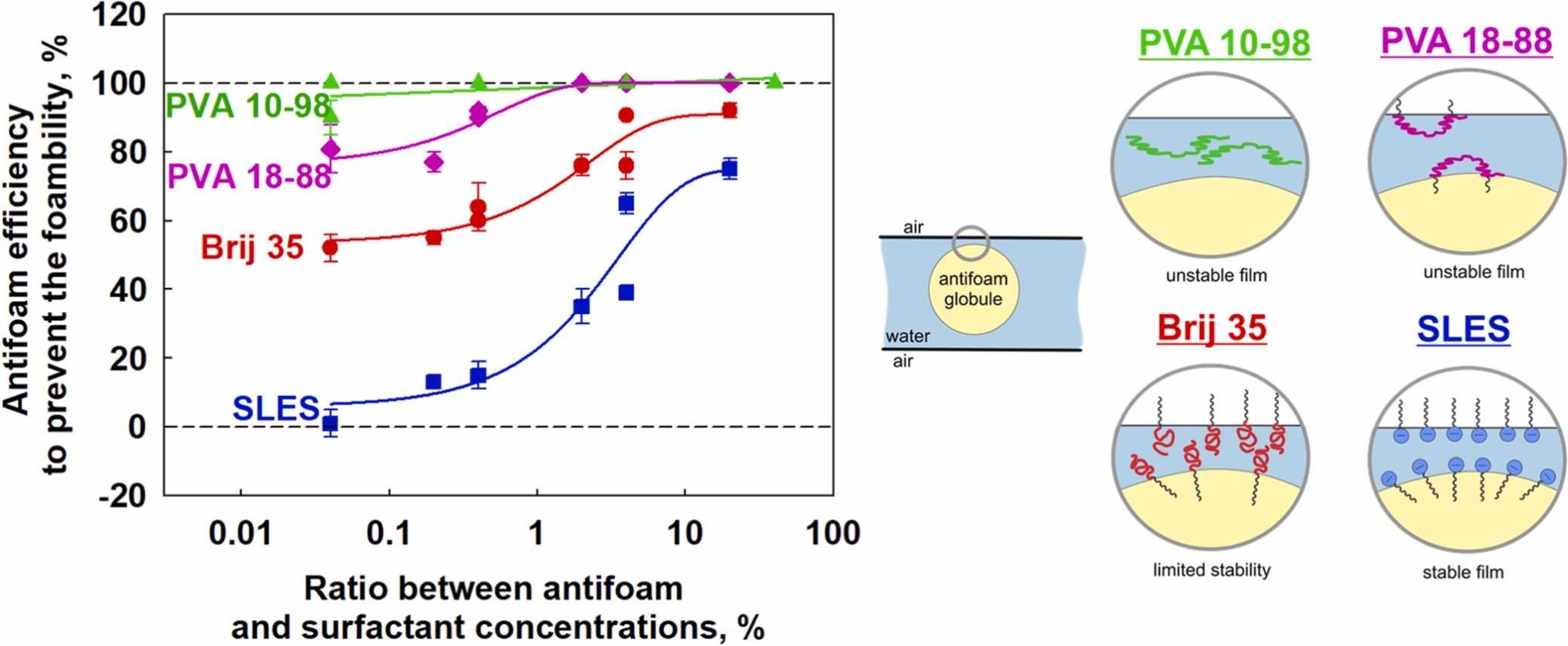
Role of hydrodynamic conditions and type of foam stabilizer for antifoam efficiency
The effects of antifoam and surfactant concentration on the foamability of solutions of an anionic (SLES) and nonionic (Brij 35) surfactants and a series of polyvinyl alcohols with 88% and 98% degree of hydrolysis and molecular masses between 31 and 205 kDa, were studied. Three methods which differ in the way of air incorporation were used for foaming – Bartsch test, shake test and Ultra Turrax. Mixed silicone oil-silica particles antifoam was studied. The antifoam was introduced in the foaming solution as pre-dispersed in organic solvent or as antifoam-in-water emulsion. It was shown that the antifoam is very active in the fast foaming methods (Bartsch and shake tests) for the slow adsorbing polymers PVA and has no any activity in the slow foaming method (Ultra Turrax) for the fast adsorbing surfactants with electrostatic stabilization (SLES). The efficiency of pre-dispersed in organic solvent antifoam is much higher as compared to that of emulsified antifoam, due to the faster segregation of the silica particles and silicone oil in the emulsified antifoam. The antifoam efficiency increases with antifoam concentration and with lowering the surfactant concentration. In a given foaming method, the antifoam efficiency is the highest in PVA solutions with 98% DH, intermediate for PVA with 88% DH and Brij 35, and the lowest for SLES solutions. At a certain degree of hydrolysis, the molecular mass of PVA has no significant effect on the antifoam activity. Good correlation between the antifoam efficiency and the stability of the pseudo emulsion film formed between the antifoam globule and the bubble surface is established, showing that the electrostatic repulsion is more efficient to prevent the entering of the antifoam globules on the air-water interface, as compared to the steric repulsion.
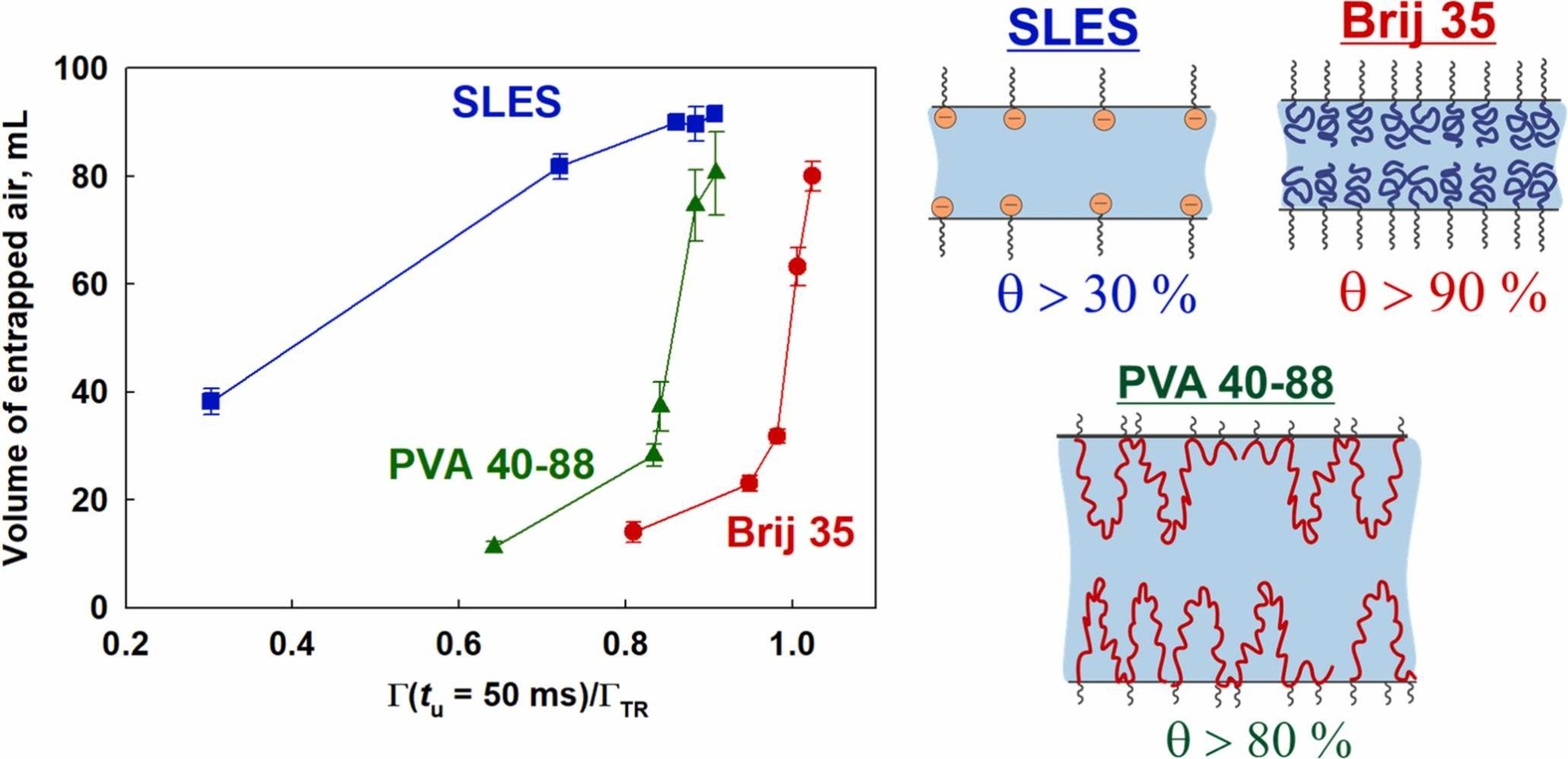
Surface and foam properties of polyvinyl alcohol solutions
The surface, film, and foam properties of six polyvinyl alcohols (PVA) with different degrees of hydrolysis (DH) and molecular weights were studied and compared with the properties of nonionic Brij 35 and anionic SLES. Four different foaming methods were employed: the fast foaming method (Bartsch test), intermediate tests (shake test and Ultra Turrax), and slow foaming method (foam rise method) to assess the foamability at various bulk concentrations. The foamability data obtained from different foaming tests, utilizing various surfactant and polymeric concentrations, and differing foaming times, were shown to follow a universal master curve when plotted as relative foamability vs. scaled concentration. A new simple theoretical equation was derived to describe this universal curve, allowing for foamability prediction. The threshold surfactant concentration required to achieve 50% of the maximal foam volume under given conditions (used for scaling the bulk concentration) was found to decrease with foaming time and increase from slow foaming methods to fast foaming methods. When the experimental data are plotted against surface coverage, the results for PVA solutions exhibit intermediate behavior between nonionic surfactants, where a threshold surface coverage of 95% is required to achieve 50% of maximal foamability and anionic surfactants, where 30% surface coverage is sufficient to reach 50% of maximal foamability due to the action of electrostatic repulsion. This intermediate behavior observed in PVA solutions is attributed to the presence of a long-range steric repulsion arising from the adsorption of PVA molecules onto the bubble surfaces. This work advances the foam field by showing that the approach developed in Petkova et al. 2020 can be used for polymeric molecules and by deriving a new equation for foamability which is expected to be applicable for wide range of systems.
Defoamer formulations containing triacylglycerides
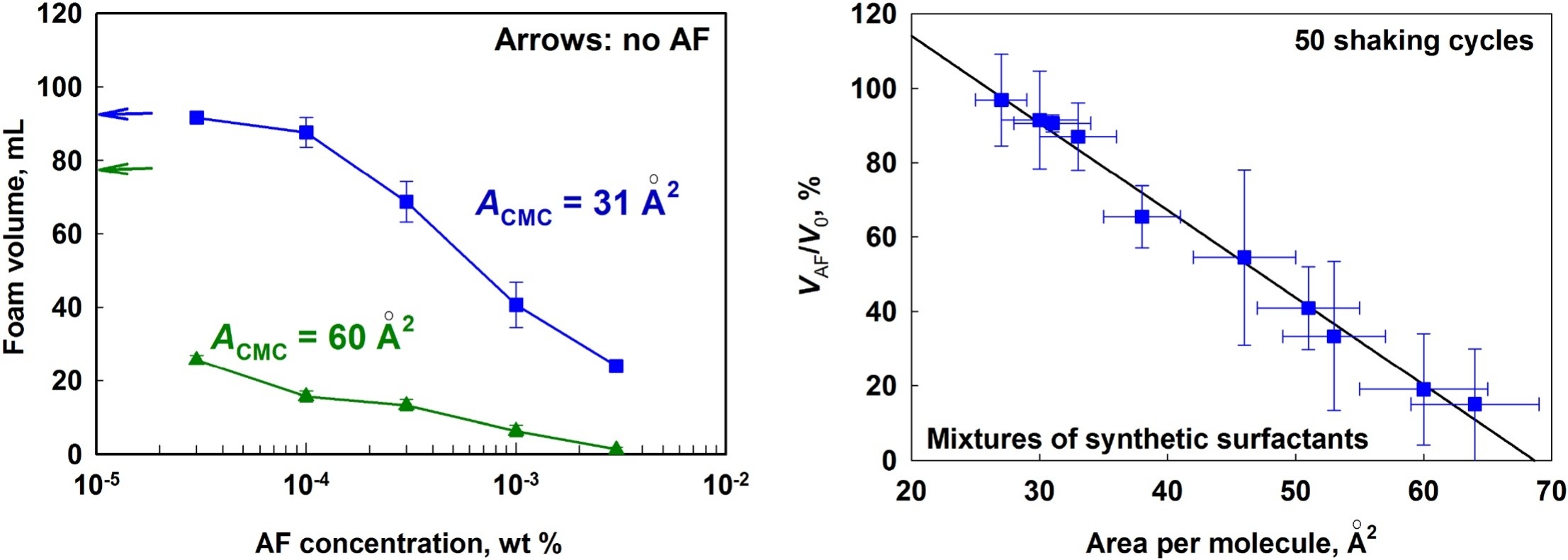
Role of surfactant adsorption and surface properties for the efficiency of PDMS-silica antifoams
We study how the composition of various surfactant mixtures affects the efficiency of mixed PDMS–silica antifoam in foamed surfactant solutions. First, systematic experiments are performed to characterize the surface and foam film properties of the studied surfactant solutions. The spreading, bridging and entry coefficients are calculated and the spreading ability of the antifoam is characterized by microscopy observations and by surface tension measurements. Next, the initial antifoam activity and the antifoam durability are characterized in foam tests. The obtained results reveal that the antifoam efficiency in solutions of low-molecular mass surfactants with low surface dilatational modulus depends strongly on the density (area-per-molecule) of the respective adsorption layer. The addition of nonionic surfactants, which increase the mean area-per-molecule in the mixed adsorption layer, enhances significantly the antifoam activity and durability. In contrast, the addition of surfactants, which decrease the mean area-per-molecule, suppresses the antifoam activity. Furthermore, we found that surfactant mixtures which form condensed adsorption layers on the solution surface suppress strongly the antifoam activity. As an extreme, the condensed adsorption layer formed from the natural surfactant Quillaja saponin suppresses the antifoam spreading even at highly positive spreading coefficient which results also in very poor AF efficiency. The obtained results rationalize in a coherent way the observed differences in the AF activity and durability in mixed solutions of various ionic, nonionic and zwitterionic surfactants.