Prof. Peter A. Kralchevsky, Ph.D., D.Sc.
Fellow of the Bulgarian Academy of Sciences
Head of the Laboratory of Complex Fluids
Interests
- Particles at Fluid Interfaces and Capillary Forces
- Capillary Phenomena, Contact Angles, Surface Forces
- General Curved Interfaces, Bending Moments, Biointerfaces
- Micelles, Solubilization and Detergency
- Thermodynamics and Kinetics of Surfactant Adsorption
Bio
M.Sc. in Physics (1981), Ph.D. (1985); Associate Professor of Chemical Physics (1991); Dr. Sci. in Physics (2001); Full Professor of Condensed Matter Physics (2002); Head of the Laboratory of Thermodynamics and Physicochemical Hydrodynamics (1993–1999); Head of the Laboratory of Chemical Physics and Engineering (2000–2008); Dean of the Faculty of Chemistry and Pharmacy (2015–2019); Head of the Laboratory of Complex Fluids (2017–present) – Sofia University, Bulgaria; Corresponding Member (2004) and Fellow (2012) of the Bulgarian Academy of Sciences. He has been visiting professor in the Illinois Institute of Technology (Chicago, USA, 1987) in the Nagayama Protein Array Project (Tsukuba, Japan, 1992); Laboratory of Ultrastructure Research, NIPS (Okazaki, Japan, 1999). His research interests are in the area of thin liquid films and surface forces, fluid interfaces, capillary forces and phenomena, phospholipid membranes, solubilization in micellar solutions, thermodynamics and kinetics of surfactant adsorption, attachment of particles to interfaces, and interactions in colloidal and bio-colloidal dispersions. So far, he has published 209 research and review papers, one book (Particles at Fluid Interfaces and Membranes, Elsevier, Amsterdam, 2001); over 10,000 citations of his publications have been found. Until now, he has delivered 262 presentations at scientific conferences: 8 plenary lectures, 50 invited and keynote lectures, 96 oral presentations and 108 posters. He has been supervisor of the Diploma Works of 39 B.Sc. and M.Sc. students and of the Theses of 16 Ph.D. students. He was the Chair of the European COST Action D43 Colloid and Interface Chemistry for Nanotechnology (2008–2011) and Vice Chair of COST Action CM1101 Colloidal Aspects of Nanoscience for Innovative Processes and Materials (2012–2016). He was recipient of the Prof. A. Zlatarov Prize of the Bulgarian Academy of Sciences and the Sofia University in 1990. He was awarded the “St. Kliment Ohridski” University of Sofia Blue Ribbon Medal for significant achievements in science (2006); the Annual award “Best Professor” (2007), and the highest National Award “Pythagoras” for scientific achievements (2016) of the Bulgarian Ministry of Education and Science. He has been Secretary of the European Colloid and Interface Society (ECIS) since 2010 and Member of the Council of the International Association of Colloid and Interface Scientists (IACIS) (2015–present).
Publications
Most recent publications
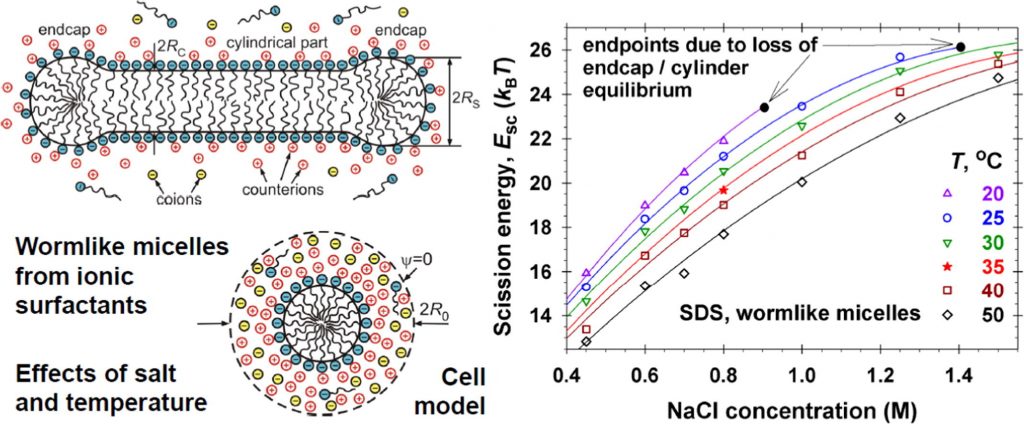
Growth of giant micellar aggregates: Quantitative theory vs experiments
The concentrated surfactant solutions have a wide application in industry, oil recovery, drug delivery, turbulent drag reduction, etc. The competition between the companies-producers has led to use of new kind of formulations to improve: washing action; skin and eye irritation; stability and durability; biodegradability; tolerance to hard water. Here, we present a review on the state of the art and our contributions to the molecular thermodynamic theory and experiment on the growth of giant micellar aggregates. Despite the considerable advances in theory and computer simulations, agreement with experimental data has been achieved only in isolated cases. Our predictive molecular thermodynamic approach accounts for the different contributions to the micellar scission energy in the case of nonionic, zwitterionic and ionic surfactant solutions and their mixtures. Excellent agreement was achieved between the theoretical model and experimental data for wormlike surfactant micelles at various concentrations of salt and temperatures. At high salt concentrations, the model also predicts loss of chemical equilibrium, which implies a transition to self-assemblies of other morphology or the onset of crystallization and phase separation. The results have applications for the design of new products and nanostructured materials.
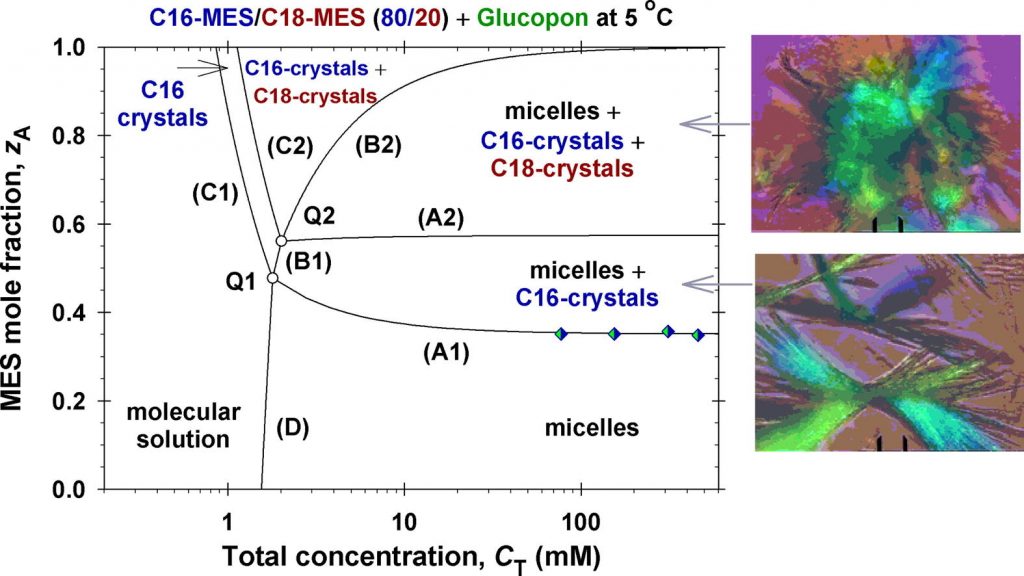
Solubility of ionic surfactants below their Krafft point in mixed micellar solutions: Phase diagrams for methyl ester sulfonates and nonionic cosurfactants
Hypothesis: Many ionic surfactants with wide applications in personal-care and house-hold detergency show limited water solubility at lower temperatures (Krafft point). This drawback can be overcome by using mixed solutions, where the ionic surfactant is incorporated in mixed micelles with another surfactant, which is soluble at lower temperatures. Experiments: The solubility and electrolytic conductivity for a binary surfactant mixture of anionic methyl ester sulfonates (MES) with nonionic alkyl polyglucoside and alkyl polyoxyethylene ether at 5 °C during long-term storage were measured. Phase diagrams were established; a general theoretical model for their explanation was developed and checked experimentally. Findings: The binary and ternary phase diagrams for studied surfactant mixtures include phase domains: mixed micelles; micelles + crystallites; crystallites, and molecular solution. The proposed general methodology, which utilizes the equations of molecular thermodynamics at minimum number of experimental measurements, is convenient for construction of such phase diagrams. The results could increase the range of applicability of MES–surfactants with relatively high Krafft temperature, but with various useful properties such as excellent biodegradability and skin compatibility; stability in hard water; good wetting and cleaning performance.
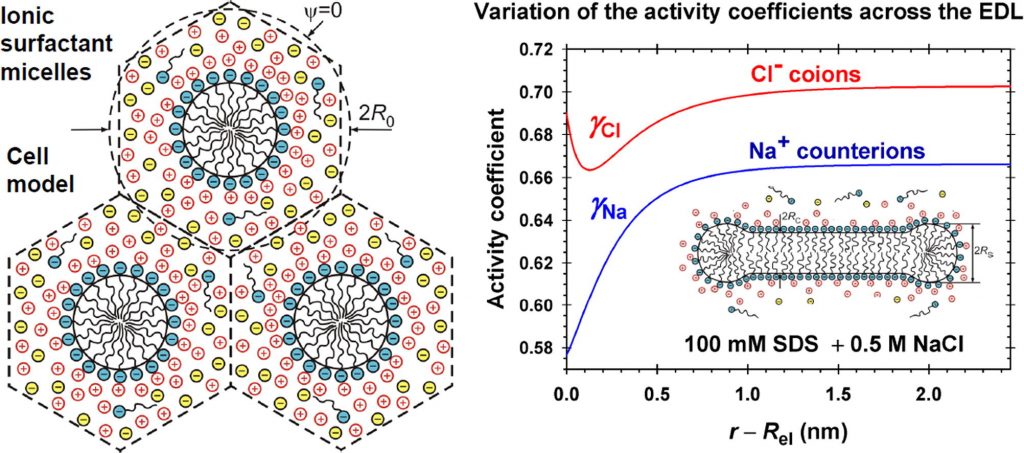
Analytical modeling of micelle growth. 3. Electrostatic free energy of ionic wormlike micelles – Effects of activity coefficients and spatially confined electric double layers
Hypotheses: To correctly predict the aggregation number and size of wormlike micelles from ionic surfactants, the molecular-thermodynamic theory has to calculate the free energy per molecule in the micelle with accuracy better than 0.01 kT, which is a serious challenge. The problem could be solved if the effects of mutual confinement of micelle counterion atmospheres, as well as the effects of counterion binding, surface curvature and ionic interactions in the electric double layer (EDL), are accurately described. Theory: The electric field is calculated using an appropriate cell model, which takes into account the aforementioned effects. Expressions for the activity coefficients have been used, which vary across the EDL and describe the electrostatic, hard sphere, and specific interactions between the ions. New approach for fast numerical calculation of the electrostatic free energy is developed. Findings: The numerical results demonstrate the variation of quantities characterizing the EDL of cylindrical and spherical micelles with the rise of electrolyte concentration. The effect of activity coefficients leads to higher values of the free energy per surfactant molecule in the micelle as compared with the case of neglected ionic interactions. The results are essential for the correct prediction of the size of wormlike micelles from ionic surfactants. This study can be extended to mixed micelles of ionic and nonionic surfactants for interpretation of the observed synergistic effects.
Analytical modeling of micelle growth. 4. Molecular thermodynamics of wormlike micelles from ionic surfactants: Theory vs. experiment
Hypotheses: The aggregation number and length of spherocylindrical (rodlike, wormlike) micelles in solutions of an ionic surfactant and salt can be predicted knowing the molecular parameters and the input concentrations of the species. This can be achieved by upgrading the quantitative molecular thermodynamic model from the previous parts of this series with an expression for the electrostatic component of micelle scission energy that is the excess free energy of the spherical endcaps with respect to the cylindrical part of the micelle. Theory: The thermodynamics of micellization is extended to the case of multicomponent system, which may contain several surfactants (both ionic and nonionic) and salts, taking into account the effect of counterion binding in the Stern layer on the micellar surface. Furthermore, the considerations are focused on a system that consists of single ionic surfactant plus salt. Findings: Excellent agreement was achieved between the theoretical model and experimental data for wormlike micelles from anionic and cationic surfactants at various concentrations of salt and temperatures. In accord with the experimental observations, at high salt concentrations, the model predicts loss of chemical equilibrium between the endcaps and cylindrical part of the wormlike micelles, which implies transition to self-assemblies of other, e.g. branched, morphology or the onset of crystallization and phase separation.