Assit. Prof. Hristina Mircheva, Ph.D. Student
Interests
- 3D Gut modeling
- Enzymatic drug degradation
Publications
Most recent publications
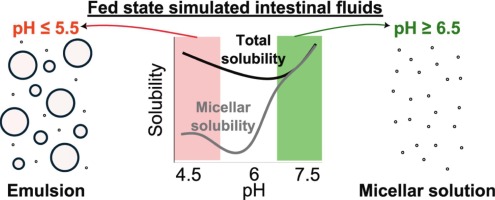
Indirect effects of pH on drug solubility in fed state simulated intestinal fluids
The pH-mediated effect of drug ionization on solubility is well-described. However, pH can also indirectly influence solubility by altering the colloidal structures in human intestinal fluids. This study investigates the indirect pH effect on the apparent solubility of 13 uncharged drugs across a pH range of 4.5 to 7.5 in fed-state simulated intestinal fluids (SIF) composed of taurocholate and lecithin, with or without added lipids (monoolein and/or sodium oleate). A pronounced indirect pH effect on drug solubility was observed when oleate was present in the SIF, whereas monoolein had only a minor effect. Below pH 6.5, sodium oleate was converted to oleic acid, resulting in lipid droplet formation that enhanced lipophilic compound solubility in the total sample (lipid phase + micellar phase), while the micellar solubility remained similar to the reference SIF (without oleate). This resulted in an up to 50-fold increase of the ratio total/micellar drug solubility, which correlated well with drug lipophilicity or its combination with total polar surface area (R2 ≈ 0.8). At higher pH, a lipid phase was not formed because the ionized sodium oleate partitioned in the micellar phase, where it significantly increased drug solubilization. These findings highlight the importance of considering indirect pH effects in solubility assessments by tuning simulated intestinal fluids composition to better reflect in vivo reality.
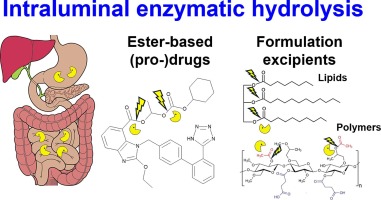
Intraluminal enzymatic hydrolysis of API and lipid or polymeric excipients
The role of intraluminal enzymes for the hydrolysis of active pharmaceutical ingredients (API), prodrugs and pharmaceutical excipients will be reviewed. Carboxylesterases may hydrolyze ester-based API, prodrugs and ester-bond containing polymer excipients, whereas lipases digest lipid formulation excipients, such as mono-, di- and triglycerides. To clarify the conditions that should be mimicked when designing in vitro studies, we briefly review the upper gastrointestinal physiology and provide new data on the inter-individual variability of enzyme activities in human intestinal fluids. Afterwards, the methodology for studying enzymatic hydrolysis of API, prodrugs, lipid and polymeric excipients, as well as the main results that have been obtained, are summarized. In vitro digestion models used to characterize lipid formulations are well described, but data about the hydrolysis of lipid excipients (including surfactants) has been scarce and contradictory. Data on API and prodrug hydrolysis by esterases is available; however, inconsistent use of enzyme types and concentrations limits structure-stability relationships. Hydrolysis of polymer excipients in the lumen has not been significantly explored, with only qualitative data available for cellulose derivates, polyesters, starches, etc. Harmonization of the methodology is required in order to curate larger enzymatic hydrolysis datasets, which will enable mechanistic understanding and theoretical prediction.