
Assoc. Prof. Diana Cholakova, Ph.D.
Interests
- Phase transitions in disperse systems
- Phase behavior of surfactants and oils
- Emulsions, Nanoparticles
- SAXS, DSC, Microscopy
Bio
Dr. Diana Cholakova completed her PhD studies in Physical Chemistry in 2020 and is currently a Post Doc at the Department of Chemical and Pharmaceutical Engineering at Sofia University, Bulgaria. Her primary research interests include phase transitions in disperse systems, phase behavior of surfactants and lipids, as well as emulsions and nanoparticles. Dr. Cholakova has co-authored 33 research papers, including three reviews published in Adv. Colloid Interface Sci. and three in Curr. Opin. Colloid Interface Sci. A microscopy image captured by Dr. Cholakova was featured as the cover of the September 2021 issue of Nature Physics. Dr. Cholakova has been recognized with several national and international awards including “Student of the Year Award” from Sofia University (2016 & 2017); the “Certificate of Achievement in Recognition of Excellence in Research” from the New York Academy of Sciences & PepsiCo (2018); “Enzo Ferroni” Award for best oral presentation by a young scientist (ECIS, 2019); “Langmuir Best Oral Presentation Award” (ECIS, 2021), and was a finalist for the “Emerging investigator award” (IACIS, 2022). In 2023, she received the prestigious national “Pythagoras” award for Best Young Scientist in the category Natural and Engineering Sciences. In 2025, she received the European Young Lipid Scientist Award by the European Federation for the Science and Technology of Lipids (Euro Fed Lipids). In June 2025, Dr. Cholakova became an Editorial Board Member in Colloids and Surfaces A: Physicochemical and Engineering Aspects journal (IF = 5.4). She has been a supervisor and co-supervisor of 6 BSc and 3 MSc students theses.
Publications
Featured publications
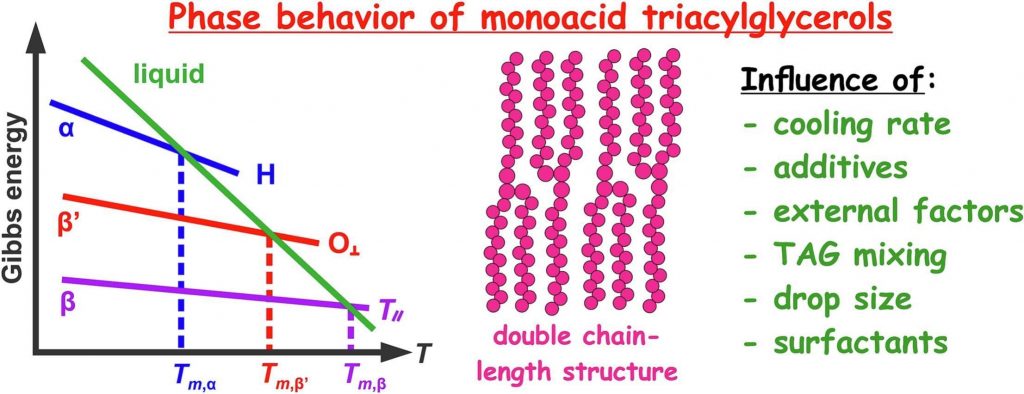
Polymorphic phase transitions in triglycerides and their mixtures studied by SAXS/WAXS techniques: In bulk and in emulsions
Triacylglycerols (TAGs) exhibit a monotropic polymorphism, forming three main polymorphic forms upon crystallization: α, β’ and β. The distinct physicochemical properties of these polymorphs, such as melting temperature, subcell lattice structure, mass density, etc., significantly impact the appearance, texture, and long-term stability of a wide range products in the food and cosmetics industries. Additionally, TAGs are also of special interest in the field of controlled drug delivery and sustained release in pharmaceuticals, being a key material in the preparation of solid lipid nanoparticles. The present article outlines our current understanding of TAG phase behavior in both bulk and emulsified systems. While our primary focus are investigations involving monoacid TAGs and their mixtures, we also include illustrative examples with natural TAG oils, highlighting the knowledge transfer from simple to intricate systems. Special attention is given to recent discoveries via X-ray scattering techniques. The main factors influencing TAG polymorphism are discussed, revealing that a higher occurrence of structural defects in the TAG structure always accelerates the rate of the α → β polymorphic transformation. Diverse approaches can be employed based on the specific system: incorporating foreign molecules or solid particles into bulk TAGs, reducing drop size in dispersed systems, or using surfactants that remain fluid during TAG particle crystallization, ensuring the necessary molecular mobility for the polymorphic transformation. Furthermore, we showcase the role of TAG polymorphism on a recently discovered phenomenon: the creation of nanoparticles as small as 20 nm from initial coarse emulsions without any mechanical energy input. This analysis underscores how the broader understanding of the TAG polymorphism can be effectively applied to comprehend and control previously unexplored processes of notable practical importance.
Rechargeable self-assembled droplet microswimmers driven by surface phase transitions
The design of artificial microswimmers is often inspired by the strategies of natural microorganisms. Many of these creatures exploit the fact that elasticity breaks the time-reversal symmetry of motion at low Reynolds numbers, but this principle has been notably absent from model systems of active, self-propelled microswimmers. Here we introduce a class of microswimmers that spontaneously self-assembles and swims without using external forces, driven instead by surface phase transitions induced by temperature variations. The swimmers are made from alkane droplets dispersed in an aqueous surfactant solution, which start to self-propel on cooling, pushed by rapidly growing thin elastic tails. When heated, the same droplets recharge by retracting their tails, swimming for up to tens of minutes in each cycle. Thermal oscillations of approximately 5 °C induce the swimmers to harness heat from the environment and recharge multiple times. We develop a detailed elasto-hydrodynamic model of these processes and highlight the molecular mechanisms involved. The system offers a convenient platform for examining symmetry breaking in the motion of swimmers exploiting flagellar elasticity. The mild conditions and biocompatible media render these microswimmers potential probes for studying biological propulsion and interactions between artificial and biological swimmers.

Self-shaping of oil droplets via the formation of intermediate rotator phases upon cooling
Revealing the chemical and physical mechanisms underlying symmetry breaking and shape transformations is key to understanding morphogenesis. If we are to synthesize artificial structures with similar control and complexity to biological systems, we need energy- and material-efficient bottom-up processes to create building blocks of various shapes that can further assemble into hierarchical structures. Lithographic top-down processing allows a high level of structural control in microparticle production but at the expense of limited productivity. Conversely, bottom-up particle syntheses have higher material and energy efficiency, but are more limited in the shapes achievable. Linear hydrocarbons are known to pass through a series of metastable plastic rotator phases before freezing. Here we show that by using appropriate cooling protocols, we can harness these phase transitions to control the deformation of liquid hydrocarbon droplets and then freeze them into solid particles, permanently preserving their shape. Upon cooling, the droplets spontaneously break their shape symmetry several times, morphing through a series of complex regular shapes owing to the internal phase-transition processes. In this way we produce particles including micrometre-sized octahedra, various polygonal platelets, O-shapes, and fibres of submicrometre diameter, which can be selectively frozen into the corresponding solid particles. This mechanism offers insights into achieving complex morphogenesis from a system with a minimal number of molecular components.
Most recent publications
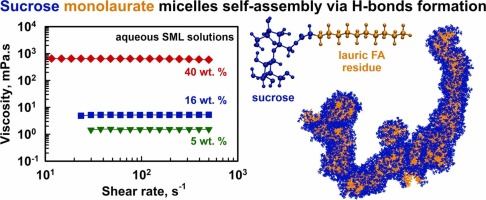
Sucrose monolaurate self-assembly via hydrogen bonding: role of surfactant concentration and urea
Sugar esters, a class of surfactants derived from renewable resources, have attracted significant attention due to their biodegradability, low toxicity, and broad applications in food, cosmetic, and pharmaceutical formulations. Despite their widespread use, the phase behavior of these compounds in aqueous systems remains incompletely understood. In this study, we investigate the self-assembly of a nonionic sucrose ester of lauric acid in 1–40 wt% concentration range using rheological measurements, dynamic light scattering, X-ray scattering, DOSY NMR, and molecular dynamics simulations. Formation of spherical micelles with a diameter of 5.4 nm is observed at low surfactant concentrations, driven by hydrophobic interactions between the alkyl tails. These solutions exhibit Newtonian flow behavior with viscosities close to that of pure water. However, the viscosity increases from 5 mPa.s at 16 wt% to 640 mPa.s at 40 wt%, while the Newtonian character persists even at 40 wt%. This behavior is explained with the formation of interconnected, thread-like micellar structures of (almost) spherical micelles that largely preserve their distinctiveness, resembling the “pearl necklace” arrangement known for polymer systems. The main driving force for this supramolecular organization was found to be the hydrogen bonding between sucrose headgroups. The addition of 6 M urea, a known hydrogen bond disruptor, significantly reduces micelle clustering and the viscosity decreases to 150 mPa.s at 40 wt% concentration, supporting the proposed aggregation mechanism. These findings contribute to a deeper understanding of the self-assembly behavior of sucrose esters in aqueous environment and highlight their potential for controlled aggregation in practical formulations.
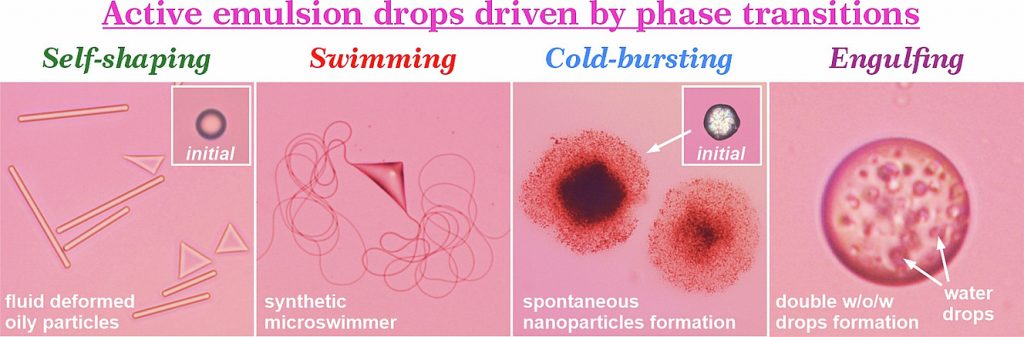
Active drops driven by surface and polymorphic phase transitions: Current understanding and emerging perspectives
Small emulsion droplets typically adopt spherical shapes under positive interfacial tension, minimizing unfavorable oil-water contact. This shape, along with the initial drop size, are generally preserved upon drop freezing or melting. However, in a series of studies, we demonstrated that simple temperature fluctuations near the melting point of the dispersed oil phase can spontaneously induce a wide range of dynamic behaviors in droplets. These activities include morphogenesis into various non-spherical shapes such as hexagonal, triangular, and tetragonal platelets, rods and fibers; the formation of complex composite micrometer-sized structures in the presence of adsorbed latex particles on initially spherical droplets; spontaneous desorption of the initially adsorbed particles; the generation of synthetic microswimmers capable of self-propulsion through the continuous phase, driven by the rapidly growing elastic filaments; spontaneous drop fragmentation and bursting into smaller particles (with sizes down to 20 nm) without any mechanical energy input; and the engulfment of the surrounding media spontaneously producing double water-in-oil-in-water droplets. All these phenomena were found to be intricately related to surface and polymorphic phase transitions proceeding within the droplets. The underlying mechanisms and control parameters were systematically investigated and published in a series of papers. The present review aims to summarize the key discoveries, present them within a unified conceptual framework, and compare them with other processes reported in the literature to lead to similar outcomes. Furthermore, the practical implications of these phenomena are discussed, and potential future research directions in this emerging area at the intersection of emulsion science and phase transition phenomena are outlined.
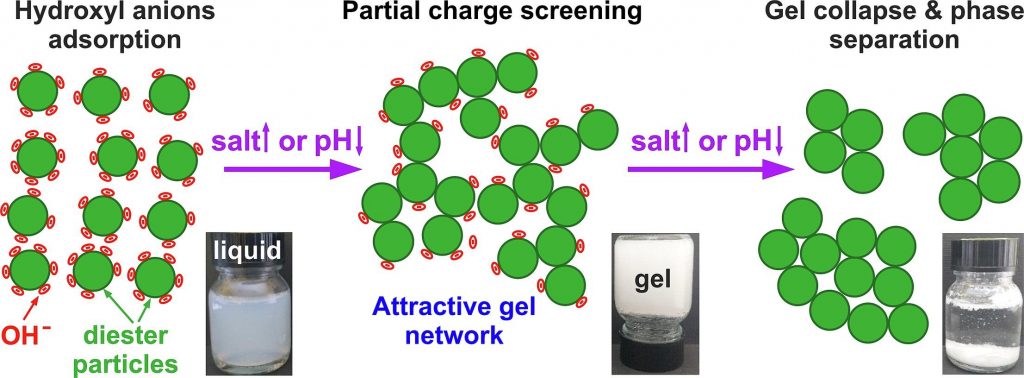
Salt-induced gelation of nonionic sucrose ester dispersions
Hypothesis
The dispersions of nonionic sucrose ester surfactants in water exhibit a highly negative zeta-potential, though its origin remains controversial. The addition of electrolytes to these dispersions may influence their zeta-potential, thus potentially affecting their physicochemical properties.
Experiments
The electrolyte- and pH- driven gelation of aqueous dispersions of commercial sucrose stearate (S970) containing ca. 1:1 monoesters and diesters was studied using optical microscopy, rheological and zeta-potential measurements, and small-angle X-ray scattering techniques.
Findings
At low electrolyte concentrations and pH ≳ 5, 0.5–5 wt% S970 dispersions exhibited low viscosities and behaved as freely flowing liquids. The addition of electrolytes of low concentrations, e.g. 9 mM NaCl or 1.5 mM MgCl2, induced the formation of a non-flowing gels. This sol–gel transition occurred due to the partial screening of the diesters particles charge, allowing the formation of an attractive gel network, spanning across the dispersion volume. Complete charge screening, however, led to a gel-sol transition and phase separation. Gel formation was observed also by pH variation without electrolyte addition, whereas the addition of free fatty acids had negligible impact on dispersion properties. These findings support the hypothesis that the negative charge in sucrose ester dispersions arises from hydroxyl anions adsorption on particles surfaces. Gels were formed using just 1.3 wt% surfactant, and the critical electrolyte concentration for gelation was found to scale approximately with the square of the cation charge, in agreement with the low surface charge density theory. The biodegradable sucrose esters gels offer a sustainable alternative for structuring personal and home care products, replacing the wormlike micelles of synthetic surfactants typically used at much higher surfactant and salt concentrations.
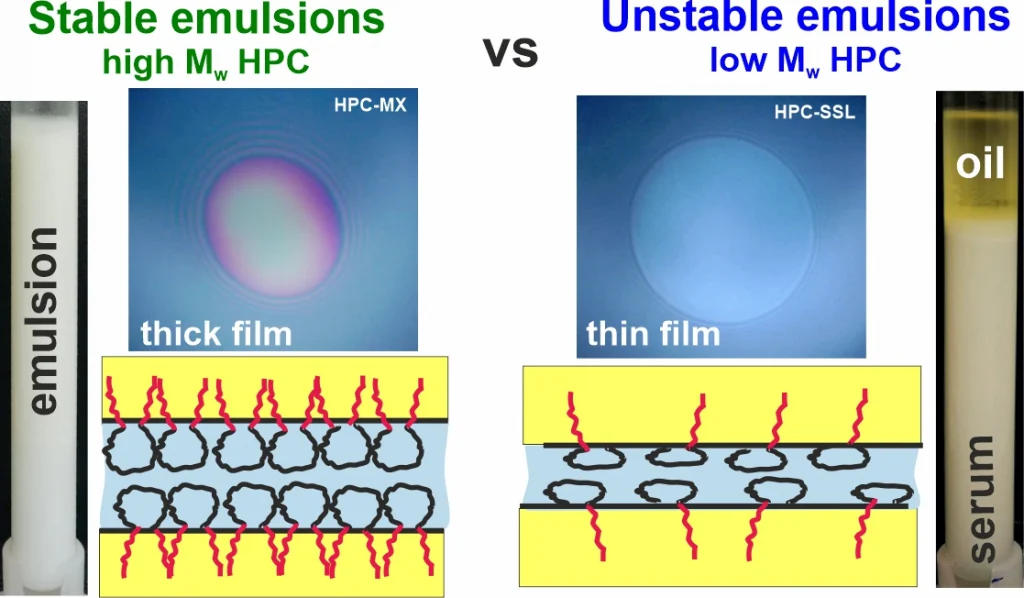
Hydroxypropyl cellulose polymers as efficient emulsion stabilizers: The effect of molecular weight and overlap concentration
Hydroxypropyl cellulose (HPC) is a non-digestible water-soluble polysaccharide used in various food, cosmetic, and pharmaceutical applications. In the current study, the aqueous solutions of six HPC grades, with molecular mass ranging from 40 to 870 kDa, were characterized with respect to their precipitation temperatures, interfacial tensions (IFTs), rheological properties and emulsifying and stabilization ability in palm (PO) and sunflower (SFO) oil emulsions. The main conclusions from the obtained results are as follows: (1) Emulsion drop size follows a master curve as a function of HPC concentration for all studied polymers, indicating that polymer molecular mass and solution viscosity have a secondary effect, while the primary effect is the fraction of surface-active molecules, estimated to be around 1–2% for all polymers. (2) Stable emulsions were obtained only with HPC polymers with Mw ≥ 400 kDa at concentrations approximately 3.5 times higher than the critical overlap concentration, c*. At PO concentrations beyond 40 wt. % or when the temperature was 25 °C, these emulsions appeared as highly viscous liquids or non-flowing gels. (3) HPC polymers with Mw < 90 kDa were unable to form stable emulsions, as the surface-active molecules cannot provide steric stabilization even at c ≳ 4–5 c*, resulting in drop creaming and coalescence during storage.
Rheology of dispersions containing non-spherical lipid particles
The rheological properties of disperse systems play a crucial role in the production of foods, cosmetics, and pharmaceuticals with desired characteristics. Emulsion viscosity can be increased through various methods, incl. increasing the oil volume fraction, incorporating rheological modifiers, or inducing partial coalescence between the droplets. It is well known that suspensions containing inorganic non-spherical particles often exhibit significantly higher viscosities when compared to those with spherical particles. The spontaneous drop self-shaping phenomenon in emulsions, first reported in detail by Denkov et al. (Nature, 2015, 528, 392–395), enables the formation of fluid and frozen lipid particles with regular non-spherical shapes, including platelets, rods and fibers. In this study, we utilize this approach to prepare emulsions containing non-spherical frozen particles of various shapes and investigate their rheological properties. The effects of oil volume fraction, surfactant type, initial drop size and polydispersity are investigated. The results reveal that non-flowing, gel-like samples can be prepared at ca. 11 vol% oil fraction when the emulsion contains polydisperse droplets which acquire non-spherical shapes upon cooling. For comparison, more than ca. 65 vol% oil is needed to obtain similar rheological characteristics in samples containing spherical particles. Additionally, we demonstrate that the optimal drop size for gel preparation is d32 ≈ 4–13 μm. The obtained results are explained mechanistically, and guiding principles are provided for preparing emulsions with increased viscosities using this new approach.


