Role of hydrodynamic conditions and type of foam stabilizer for antifoam efficiency
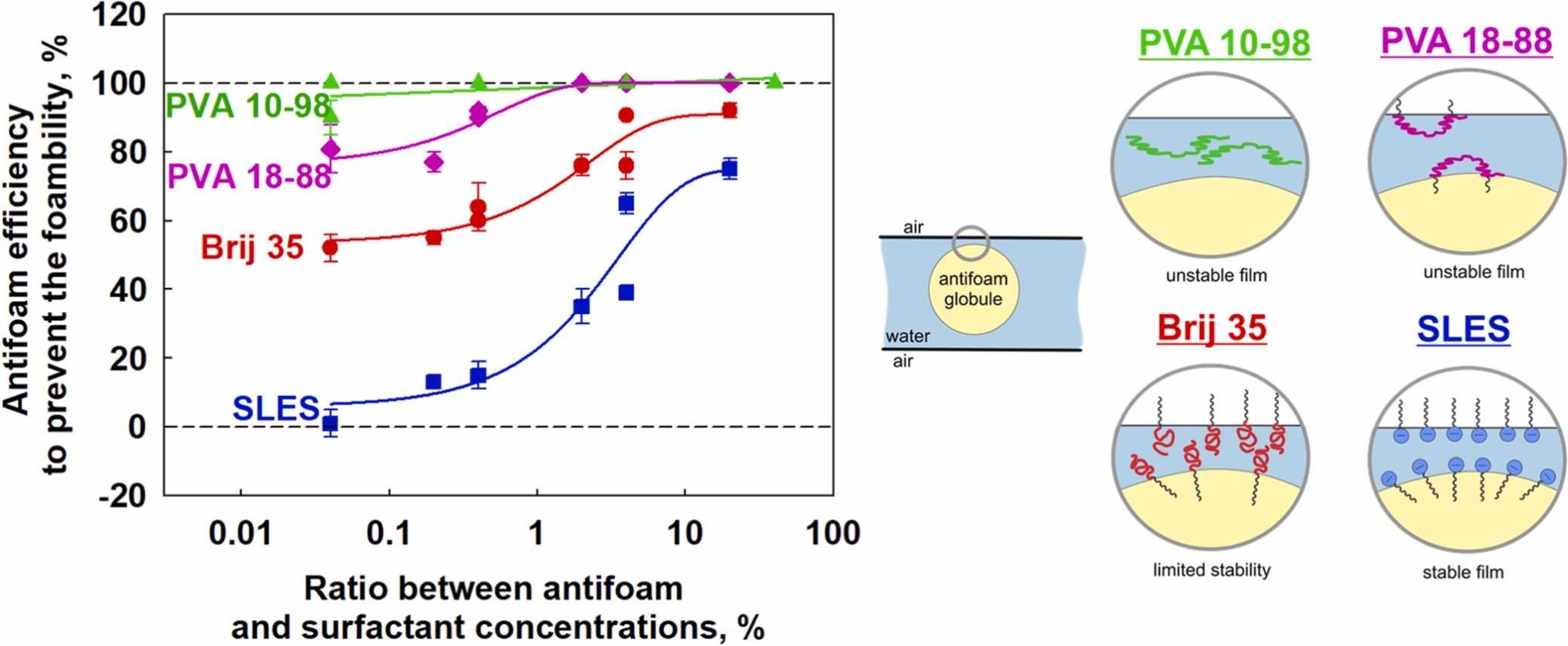
The effects of antifoam and surfactant concentration on the foamability of solutions of an anionic (SLES) and nonionic (Brij 35) surfactants and a series of polyvinyl alcohols with 88% and 98% degree of hydrolysis and molecular masses between 31 and 205 kDa, were studied. Three methods which differ in the way of air incorporation were used for foaming – Bartsch test, shake test and Ultra Turrax. Mixed silicone oil-silica particles antifoam was studied. The antifoam was introduced in the foaming solution as pre-dispersed in organic solvent or as antifoam-in-water emulsion. It was shown that the antifoam is very active in the fast foaming methods (Bartsch and shake tests) for the slow adsorbing polymers PVA and has no any activity in the slow foaming method (Ultra Turrax) for the fast adsorbing surfactants with electrostatic stabilization (SLES). The efficiency of pre-dispersed in organic solvent antifoam is much higher as compared to that of emulsified antifoam, due to the faster segregation of the silica particles and silicone oil in the emulsified antifoam. The antifoam efficiency increases with antifoam concentration and with lowering the surfactant concentration. In a given foaming method, the antifoam efficiency is the highest in PVA solutions with 98% DH, intermediate for PVA with 88% DH and Brij 35, and the lowest for SLES solutions. At a certain degree of hydrolysis, the molecular mass of PVA has no significant effect on the antifoam activity. Good correlation between the antifoam efficiency and the stability of the pseudo emulsion film formed between the antifoam globule and the bubble surface is established, showing that the electrostatic repulsion is more efficient to prevent the entering of the antifoam globules on the air-water interface, as compared to the steric repulsion.

