Characterization of neonatal and infant enterostomy fluids – Part II: Drug solubility
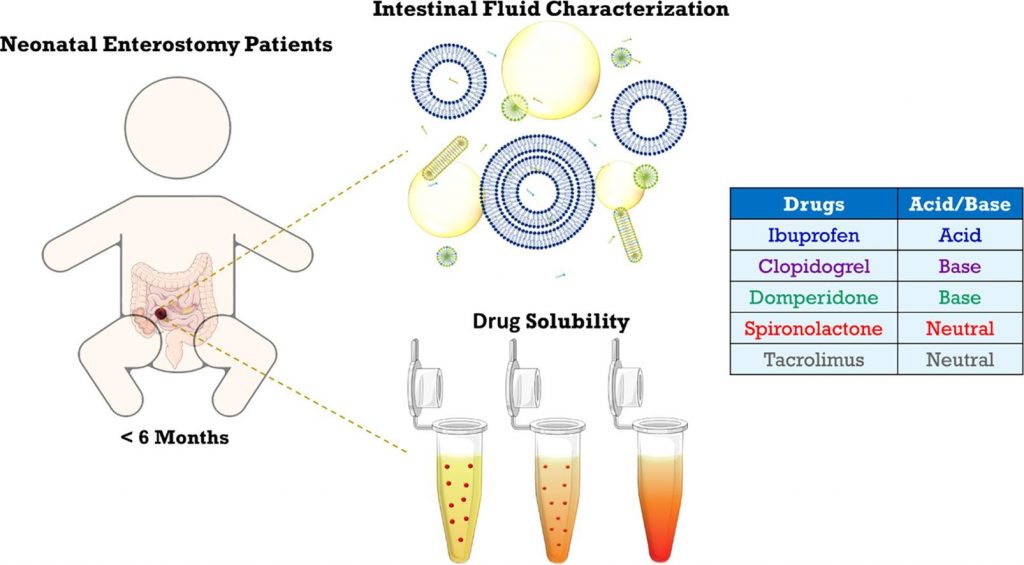
Previous research revealed marked differences in the composition of intestinal fluids between infants and adults. To explore the impact on the solubilization of orally administered drugs, the present study assessed the solubility of five poorly water-soluble, lipophilic drugs in intestinal fluid pools from 19 infant enterostomy patients (infant HIF). For some but not all drugs, the average solubilizing capacity of infant HIF was similar to that of HIF obtained from adults (adult HIF) in fed conditions. Commonly used fed state simulated intestinal fluids (FeSSIF(-V2)) predicted fairly well drug solubility in the aqueous fraction of infant HIF, but did not account for the substantial solubilization by the lipid phase of infant HIF. Despite similarities in the average solubilities of some drugs in infant HIF and adult HIF or SIF, the underlying solubilization mechanisms likely differ, considering important compositional differences (e.g., low bile salt levels). Finally, the huge variability in composition of infant HIF pools resulted in a highly variable solubilizing capacity, potentially causing variations in drug bioavailability. The current study warrants future research focusing on (i) understanding the mechanisms underlying drug solubilization in infant HIF and (ii) evaluating the sensitivity of oral drug products to interpatient variations in drug solubilization.

