Veronika I. Yavrukova (Ivanova), Ph.D.
Interests
- Surfactant layers morphology on substrates by ellypsometry
- Wetting of surfactant layers
- Static and Dynamic Surface Tension
- Micellization and Rheology of Surfactant Solutions
- Protein Adsorption on Solid Surfaces by AFM
- Laundry formulation
- Cleaning formulations
- Phase diagrams
Publications
Most recent publications
Investigation of the detergency properties of mixtures of biocides and nonionic surfactants using a new simplified hard surface cleaning method
The present study explores the cleaning efficacy of a set of nonionic surfactants (linear ethoxylated alcohol, secondary ethoxylated alcohols with 5, 7, and 9 ethoxy groups, glycoside surfactants, polyglycerol surfactants, and an ethoxylated sorbitan monolaurate) combined with cationic biocides—alkyl quaternary ammonium salts. A simple hard surface cleaning methodology was applied, which was shown to discriminate well between poor and good cleaning formulations. In addition to cleaning efficacy, surface aesthetics such as gloss and haze were evaluated together to assess surface streaking caused by a residual surfactant layer. The haziness determination turned out to be the key feature revealing the complex cleaning performance of multi-component products.

Enhanced solubility of methyl ester sulfonates below their Krafft points in mixed micellar solutions
Hypothesis: Methyl ester sulfonates (MES) show limited water solubility at lower temperatures (Krafft point). One way to increase their solubility below their Krafft points is to incorporate them in anionic surfactant micelles. The electrostatic interactions between the ionic surfactant molecules and charged micelles play an important role for the degree of MES solubility. Experiments: The solubility and electrolytic conductivity for binary and ternary surfactant mixtures of MES with anionic sodium alpha olefin sulfonate (AOS) and sodium lauryl ether sulfate with two ethylene oxide groups (SLES-2EO) at 5 °C during long-term storage were measured. Phase diagrams were established; a general phase separation theoretical model for their explanation was developed and checked experimentally. Findings: The binary and ternary phase diagrams for studied surfactant mixtures include phase domains: mixed micelles; micelles + crystallites; crystallites, and molecular solution. The proposed general phase separation model for ionic surfactant mixtures is convenient for construction of such complex phase diagrams and provides information on the concentrations of all components of the complex solution and on the micellar electrostatic potential. The obtained maximal MES mole fraction of transparent micellar solutions could be of interest to increase the range of applicability of MES–surfactants.
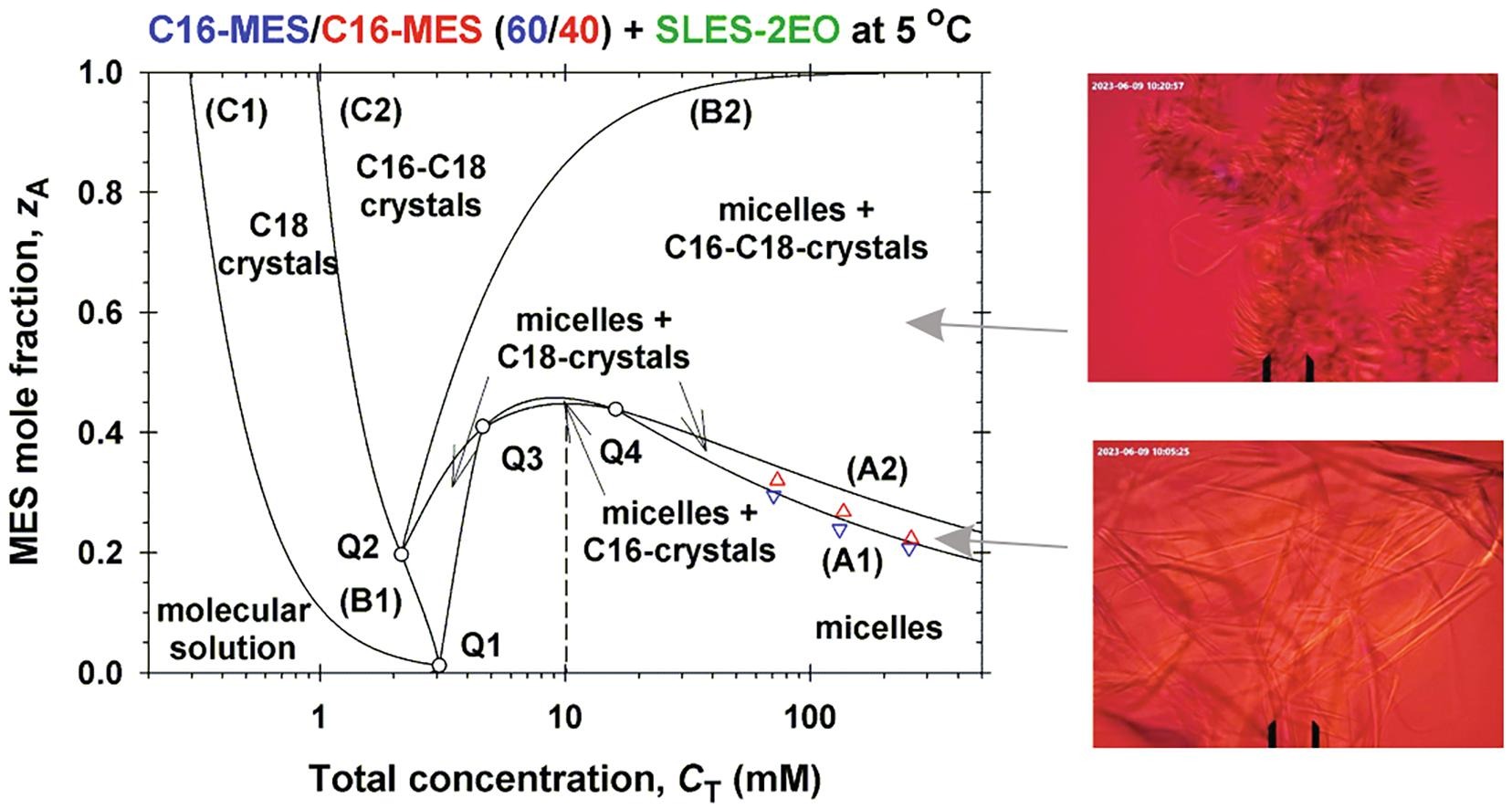
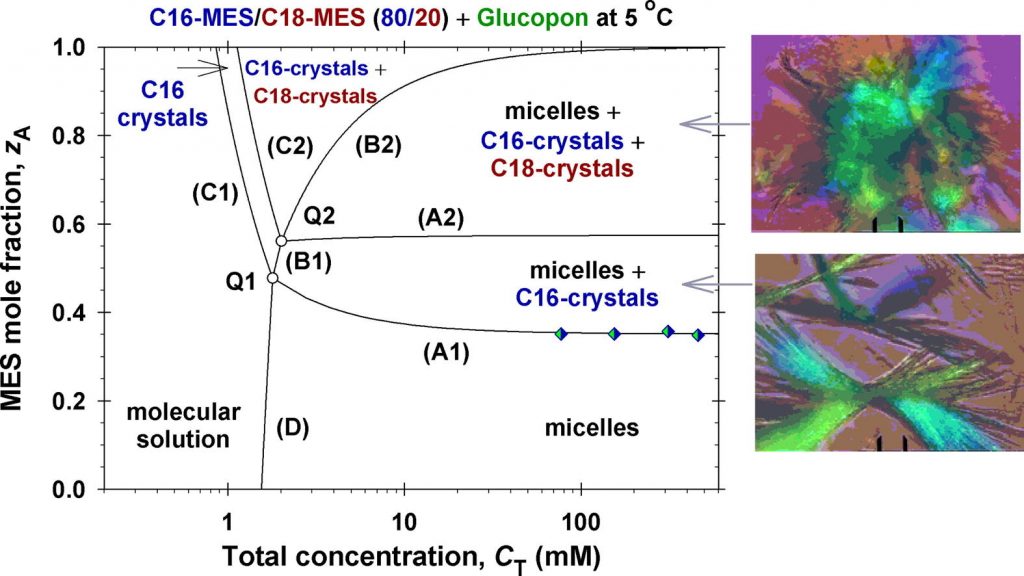
Solubility of ionic surfactants below their Krafft point in mixed micellar solutions: Phase diagrams for methyl ester sulfonates and nonionic cosurfactants
Hypothesis: Many ionic surfactants with wide applications in personal-care and house-hold detergency show limited water solubility at lower temperatures (Krafft point). This drawback can be overcome by using mixed solutions, where the ionic surfactant is incorporated in mixed micelles with another surfactant, which is soluble at lower temperatures. Experiments: The solubility and electrolytic conductivity for a binary surfactant mixture of anionic methyl ester sulfonates (MES) with nonionic alkyl polyglucoside and alkyl polyoxyethylene ether at 5 °C during long-term storage were measured. Phase diagrams were established; a general theoretical model for their explanation was developed and checked experimentally. Findings: The binary and ternary phase diagrams for studied surfactant mixtures include phase domains: mixed micelles; micelles + crystallites; crystallites, and molecular solution. The proposed general methodology, which utilizes the equations of molecular thermodynamics at minimum number of experimental measurements, is convenient for construction of such phase diagrams. The results could increase the range of applicability of MES–surfactants with relatively high Krafft temperature, but with various useful properties such as excellent biodegradability and skin compatibility; stability in hard water; good wetting and cleaning performance.
Cleaning ability of mixed solutions of sulfonated fatty acid methyl esters
Here, we present results from a systematic study on cleaning of oily deposits from solid surfaces (porcelain and stainless steel) by solutions of fatty acid sulfonated methyl esters (SME), sodium salts. The zwitterionic dodecyldimethylamine oxide (DDAO) has been used as a cosurfactant. As representatives of the vegetable and mineral oils, sunflower seed oil and light mineral oil have been used. The process of oil drop detachment from the solid substrates (roll-up mechanism) has been monitored. In the case of porcelain, excellent cleaning of oil is achieved by mixed solutions of SME and DDAO. In the case of stainless steel, excellent cleaning (superior than that by linear alkylbenzene sulfonate and sodium lauryl ether sulfate) is provided by binary and ternary mixtures of SME, which may contain also DDAO. For the studied systems, the good cleaning correlates neither with the oil/water interfacial tension, nor with the surfactant chainlength and headgroup type. The data imply that governing factors might be the thickness and morphology of admicelle layers formed on the solid/water interface. The results indicate that the SME mixtures represent a promising system for formulations in house-hold detergency, having in mind also other useful properties of SME, such as biodegradability, skin compatibility, and hard water tolerance.
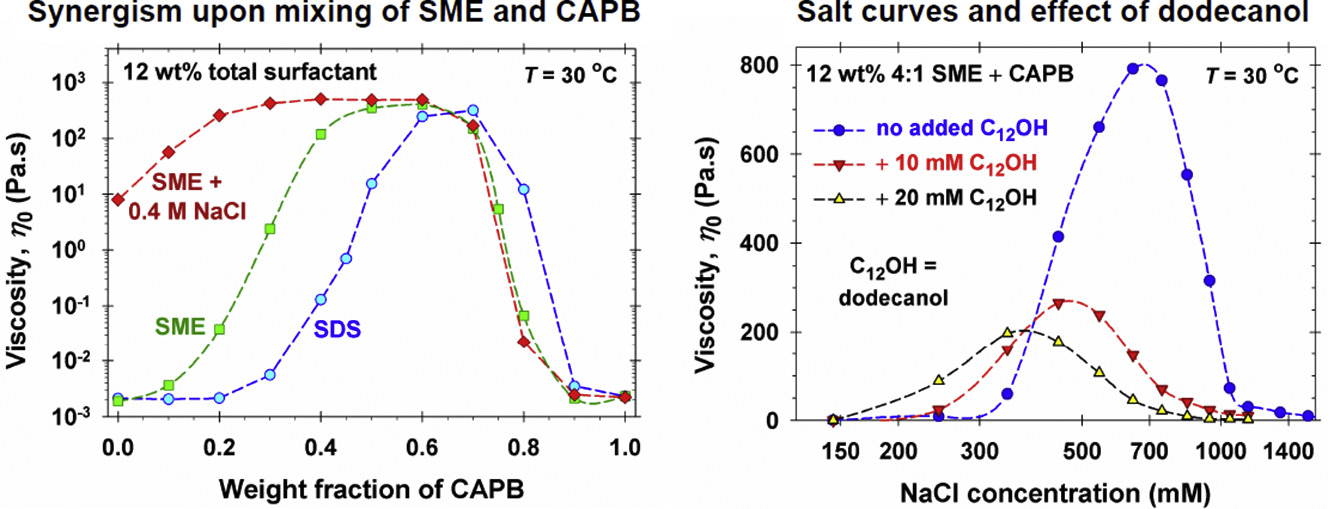
Rheology of mixed solutions of sulfonated methyl esters and betaine in relation to the growth of giant micelles and shampoo applications
This is a review article on the rheological properties of mixed solutions of sulfonated methyl esters (SME) and cocamidopropyl betaine (CAPB), which are related to the synergistic growth of giant micelles. Effects of additives, such as fatty alcohols, cocamide monoethanolamine (CMEA) and salt, which are expected to boost the growth of wormlike micelles, are studied. We report and systematize the most significant observed effects with an emphasis on the interpretation at molecular level and understanding the rheological behavior of these systems. The experiments show that the mixing of SME and CAPB produces a significant rise of viscosity, which is greater than in the mixed solutions of sodium dodecyl sulfate and CAPB. The addition of fatty alcohols, CMEA and cationic polymer, leads to broadening of the synergistic peak in viscosity without any pronounced effect on its height. The addition of NaCl leads to a typical salt curve with high maximum, but in the presence of dodecanol this maximum is much lower. At lower salt concentrations, the fatty alcohol acts as a thickener, whereas at higher salt concentrations – as a thinning agent. Depending on the shape of the frequency dependences of the measured storage and loss moduli, G’ and G”, the investigated micellar solutions behave as systems of standard or nonstandard rheological behavior. The systems with standard behavior obey the Maxwell viscoelastic model (at least) up to the crossover point (G’ = G”) and can be analyzed in terms of the Cates reptation-reaction model. The systems with nonstandard rheological behavior obey the Maxwell model only in a restricted domain below the crossover frequency; they can be analyzed in the framework of an augmented version of the Maxwell model. The methodology for data analysis and interpretation could be applied to any other viscoelastic micellar system.