Prof. Theodor D. Gurkov, Ph.D.
Interests
- Mechanics and thermodynamics of liquid interfaces and membranes
- Statistical thermodynamics of colloid systems
- Interfacial and capillary phenomena
- Thermodynamics and kinetics of adsorption, surface equation of state
- Surface rheology (viscoelasticity, yield stress)
- Stability of liquid dispersions (emulsions, foams, role of surfactants, proteins, particles)
- Thin liquid films (interactions and hydrodynamics)
- Micro-heterogeneous systems
- Contact angles and wetting
Bio
M.Sc. in Chemistry, Sofia University (1984), Ph.D. in Physical Chemistry (1997). Assistant Professor (1998). Associate Proffesor of Physical Chemistry (2002); Professor in Theoretical Chemistry (2016). Visiting researcher in Ecole Normale Superieure, Paris, France (1993); Lehrstuhl für Strömungsmechanik, Universität Erlangen-Nürnberg, Germany (1995); Lab. Ultrastructure Res., Natl. Inst. Physiological Sci., Okazaki, Japan (2001). He has published 57 scientific research papers in the fields of: mechanics and thermodynamics of fluid interfaces and membranes; interfacial phenomena and adsorption; surface rheology; bulk rheology of complex fluids; disperse systems in presence of proteins, polymers, particles – stability of emulsions and foams, including statistical aspects; thin liquid films – interactions and stability; hydrodynamics of micro-heterogeneous systems. He has presented over 26 contributions at international conferences and symposia (from the year 2000); has delivered invited lectures in Germany, Israel.
Publications
Most recent publications
Quantitative characterization of the mass transfer of volatile amphiphiles between vapor and aqueous phases: Experiment vs theory
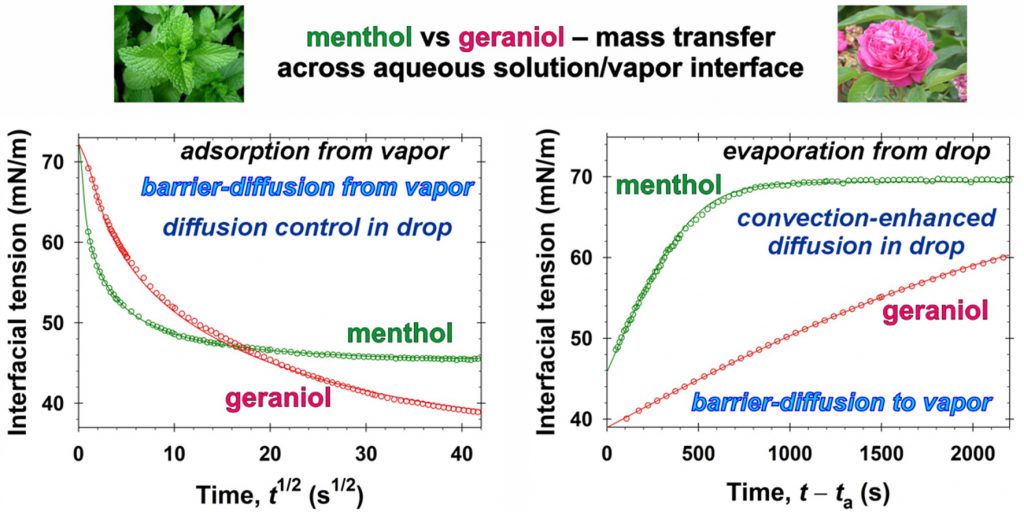
The class of volatiles, which possess low saturated vapor pressures, appreciable solubilities in water, and well pronounced surface activities, have gained wide applications in diverse areas of industry, cosmetics, and medicine. One way to qualitatively characterize their mass transfer between vapor and aqueous solutions is to measure the relaxation of the interfacial tension, σ, with time, t, under different nonequilibrium initial conditions. This approach is applied in the present work for geraniol and menthol. By means of combining σ(t) data with the respective equilibrium surface tension isotherms, the instantaneous values of the fragrance adsorption, Γ(t), have been determined. Quantitative characterization of the geraniol and menthol mass transfers in the case of adsorption from vapor to aqueous drops is achieved by using a mixed barrier-diffusion model. The obtained values of the rates of adsorption and desorption are compared with those reported in the literature for benzyl acetate, linalool, and citronellol. In the case of evaporation of the volatiles from their saturated aqueous solutions to the ambient atmosphere, the mass transfer is found to be driven both by mixed barrier-diffusion and by convection-enhanced mechanisms – depending on the air humidity. The quantitative description of the evaporation of volatile molecules is modelled theoretically by adsorption rate constants. In order to achieve the reported model representations, complex numerical calculations are implemented. On the other hand, having in mind the cases when one wishes to avoid extensive computational work, we developed a simple semiempirical model suitable for all five studied fragrances. This simplified approach is convenient for the express comparison and characterization of the evaporation rates. The obtained physicochemical parameters related to the evaporation and condensation of volatiles are important for the rigorous modeling of their complex mixed solutions of practical interest. The semiempirical model could be used for the quantitative classification of volatile molecules with respect to their ability to evaporate.
Volatile aroma surfactants: The evaluation of the adsorption-evaporation behavior under dynamic and equilibrium conditions
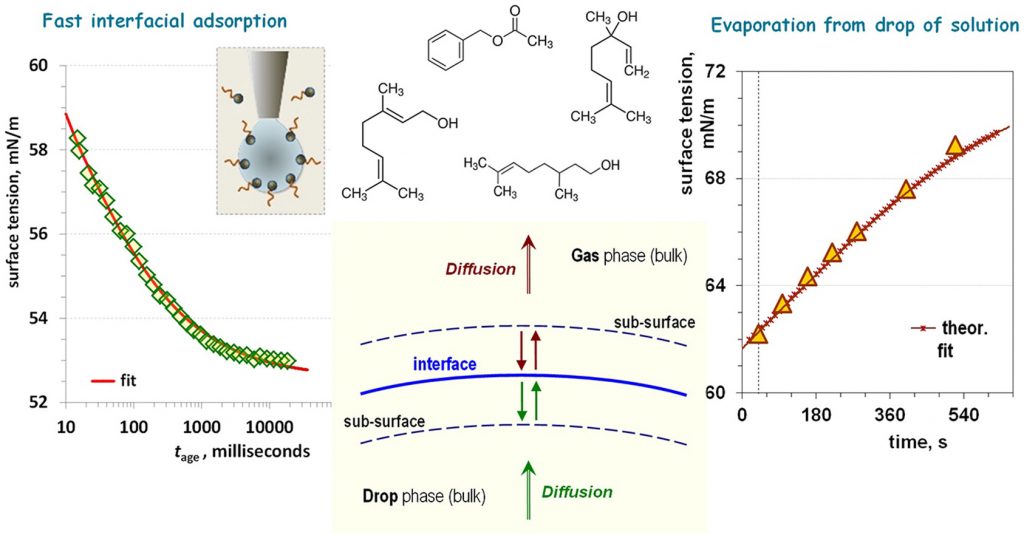
Multicomponent heterogeneous systems containing volatile amphiphiles are relevant to the fields ranging from drug delivery to atmospheric science. Research presented here discloses the individual interfacial activity and adsorption-evaporation behavior of amphiphilic aroma molecules at the liquid-vapor interface. The surface tension of solutions of nonmicellar volatile surfactants linalool and benzyl acetate, fragrances as such, was compared with that of the conventional surfactant sodium dodecyl sulfate (SDS) under equilibrium as well as under no instantaneous equilibrium, including a fast-adsorbing regime. In open systems, the increase in the surface tension on a time scale of ∼10 min is evaluated using a phenomenological model. The derived characteristic mass transfer constant is shown to be specific to both the desorption mechanism and the chemistry of the volatile amphiphile. Fast-adsorbing behavior disclosed here, as well as the synergetic effect in the mixtures with conventional micellar surfactants, justifies the advantages of volatile amphiphiles as cosurfactants in dynamic interfacial processes. The demonstrated approach to derive specific material parameters of fragrance molecules can be used for an application-targeted selection of volatile cosurfactants, e.g., in emulsification and foaming, inkjet printing, microfluidics, spraying, and coating technologies.
Volatile surfactants: Characterization and areas of application
Amphiphilic aroma molecules, representatives of fragrance molecules, are introduced as dynamic volatile surfactants. Surface tension of their aqueous solutions proves to be a sensitive and revealing quantity, used for assessment of the adsorption-evaporation behavior both under equilibrium conditions and in regimes of no instantaneous equilibrium. Such volatile amphiphiles are characterized by fast adsorption from bulk solution at an air-water interface, on a timescale of tens of microseconds, and exhibit synergetic effect in mixtures with conventional micellar-forming surfactants. Their ability to evaporate from the interface on a time scale of minutes suggests their applications as “temporal” dynamic cosurfactants in technologies involving fast formation of new surfaces. Current challenges concern evaluation of specific material parameters of volatile aroma surfactants in order to enable their selection for targeted applications.
Kinetics of transfer of volatile amphiphiles (fragrances) from vapors to aqueous drops and vice versa: Interplay of diffusion and barrier mechanisms
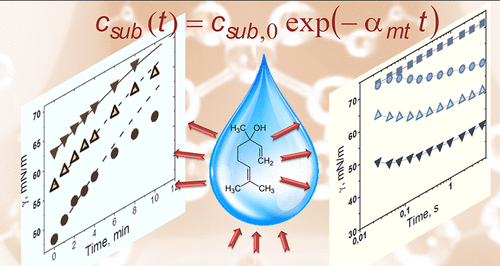
Subject of this work is to investigate the kinetics of mass transfer of volatile amphiphiles from their vapors to aqueous drops, and from the saturated aqueous drop solutions to air. The used amphiphiles are benzyl acetate, linalool, and citronellol, all of which have low saturated vapor pressures, appreciable solubility in water, and well pronounced surface activity. The adequate theoretical processing of the equilibrium surface tension, σ, isotherms is applied to construct the two-dimensional equation of state, which relates σ to the adsorption, Γ, at the interface. The measured surface tension relaxations with time t in the regimes of adsorption from vapor and evaporation from drop combined with the equations of state provide quantitative information on the change of adsorption because of the volatile amphiphile mass transfer across the surface. The theoretical analysis of the diffusion and barrier mechanisms in the case of adsorption from vapor to the aqueous drop shows that the mixed barrier-diffusion control in the vapor and diffusion control in the drop describe experimental data. The obtained values of the adsorption rate constants are six orders of magnitude larger than those for hexane and cyclohexane reported in the literature. The regime of evaporation from aqueous amphiphile solution drop follows the convection-enhanced adsorption mechanism with desorption rate constant in the vapor affected by the simultaneous water evaporation and amphiphile desorption. The water evaporation suppresses the evaporation of linalool and accelerates desorption of benzyl acetate and citronellol. From viewpoint of applications, the obtained physicochemical parameters of the studied three fragrances can help for better understanding of their performance in shampoo systems and perfumes. From theoretical viewpoint, the result show that by introducing an effective amphiphile desorption rate constant it is possible to quantify the complex volatile amphiphile desorption accompanied with the water evaporation.
 from vapors to aqueous drops and vice versa-Interplay of diffusion and barrier mechanisms-1024x782.jpg)
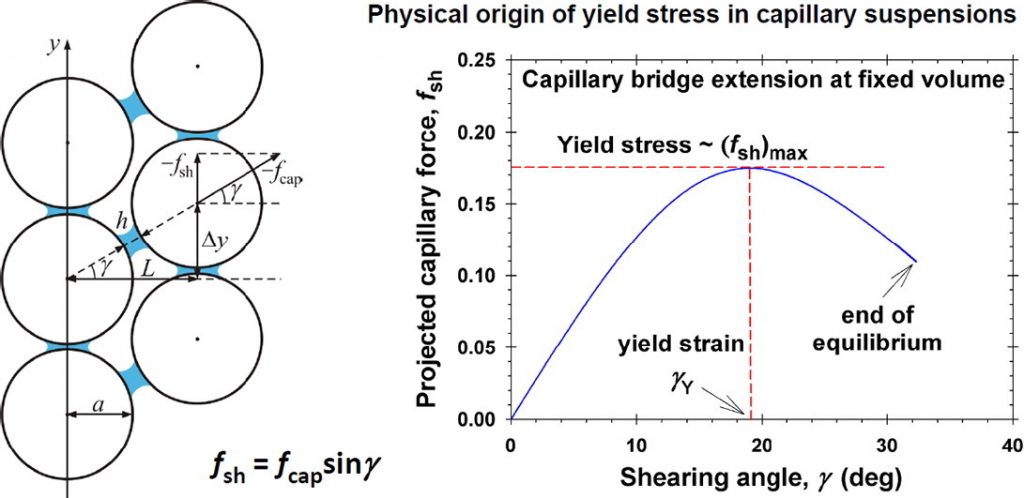
Hardening of particle/oil/water suspensions due to capillary bridges: Experimental yield stress and theoretical interpretation
Suspensions of colloid particles possess the remarkable property to solidify upon the addition of minimal amount of a second liquid that preferentially wets the particles. The hardening is due to the formation of capillary bridges (pendular rings), which connect the particles. Here, we review works on the mechanical properties of such suspensions and related works on the capillary-bridge force, and present new rheological data for the weakly studied concentration range 30–55 vol% particles. The mechanical strength of the solidified capillary suspensions, characterized by the yield stress Y, is measured at the elastic limit for various volume fractions of the particles and the preferentially wetting liquid. A quantitative theoretical model is developed, which relates Y with the maximum of the capillary-bridge force, projected on the shear plane. A semi-empirical expression for the mean number of capillary bridges per particle is proposed. The model agrees very well with the experimental data and gives a quantitative description of the yield stress, which increases with the rise of interfacial tension and with the volume fractions of particles and capillary bridges, but decreases with the rise of particle radius and contact angle. The quantitative description of capillary force is based on the exact theory and numerical calculation of the capillary bridge profile at various bridge volumes and contact angles. An analytical formula for Y is also derived. The comparison of the theoretical and experimental strain at the elastic limit reveals that the fluidization of the capillary suspension takes place only in a deformation zone of thickness up to several hundred particle diameters, which is adjacent to the rheometer’s mobile plate. The reported experimental results refer to water-continuous suspension with hydrophobic particles and oily capillary bridges. The comparison of data for bridges from soybean oil and hexadecane surprisingly indicate that the yield strength is greater for the suspension with soybean oil despite its lower interfacial tension against water. The result can be explained with the different contact angles of the two oils in agreement with the theoretical predictions. The results could contribute for a better understanding, quantitative prediction and control of the mechanical properties of three-phase capillary suspensions solid/liquid/liquid.