Tatiana Slavova, PhD Student
Interests
- Static and Dynamic Surface Tension
- Emulsification, encapsulation, core-in-shell structures
- Contact angles and wetting; effect of surfactants and polymers
- Cleaning, attachment/detachment of oil drops
Publications
Most recent publications
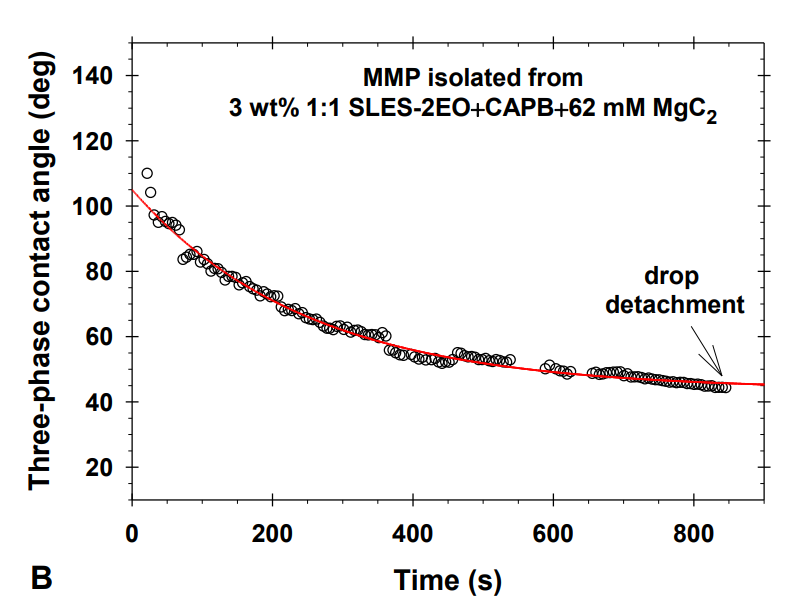
Multiconnected micellar phases for cleaning applications
The research paper explores the ability of isolated multiconnected micellar phases (MMP) to remove different soils (dimethicone, sebum oil, and makeup formulations) from artificial skin. The MMP is isolated from 3 wt% 1:1 sodium lauryl ether sulfate with 2 ethylene oxide group + cocamidopropyl betaine in the presence of 62 mM MgCl2. The direct observations of soil removal demonstrate the efficacy of the MMP by two physicochemical mechanisms. For dimethicone soils, the roll-up mechanism is observed, i.e. shrinkages of the drop contact areas and decrease of the three-phase contact angles due to the changes in the interfacial tension and the surface energy. For sebum soils, the spontaneous emulsification mechanism takes place because of the mixing of the fatty acids present in the sebum with the surfactants and forming complex amphiphilic structures. For makeup formulations, the new approach based on the software image analysis by means of Image J is developed to account for the whole soiled area and to increase the precision of the quantitative cleaning characterization. The obtained results show that the bicontinuous phase successfully removes dimethicone, sebum oil, and foundation soils from the artificial skin and could have potential application in the design of new personal, home and industrial care products.
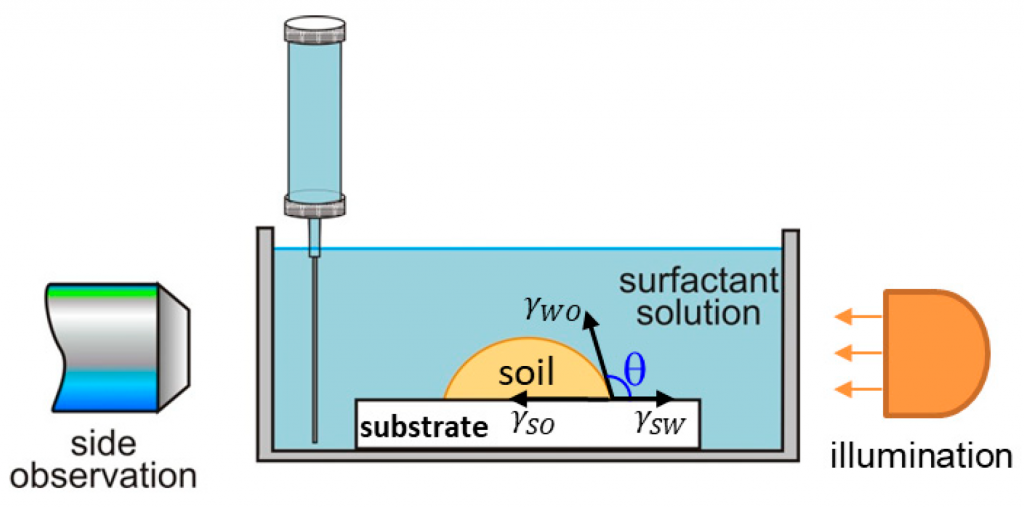
Cleansing mechanisms and efficacy on artificial skin
A systematic study on the mechanisms of cleansing artificial skin by solutions of widely used in personal care surfactants disodium laureth sulfosuccinate (DSLSS), sodium laureth sulfate (SLES), sodium dodecyl sulfate (SDS), dodecyl trimethyl ammonium bromide (DTAB), and coco glucoside (CG), is presented. The systematic characterization of soil removal from artificial skin revealed two primary cleansing mechanisms: emulsification and roll-up. Emulsification occurs in systems with very low interfacial tension, such as sebum in SLES solutions, while dimethicone soil was only removed by roll-up. The roll-up effectiveness depends on the surfactant’s interfacial activity and its adsorption on the soiled surface. Thus, the strong adsorption of DTAB on the skin leads to dimethicone roll-up at a relatively high interfacial tension of 11 mN/m. The anionic and nonionic surfactants adsorbed less at the artificial skin surface, and the oil/water interfacial tension value lowering below 5 mN/m is necessary for the roll-up to occur. Nonionic CG removed dimethicone at a lower concentration than ionic surfactants. Combining CG with ionic surfactants improved cleaning at lower total concentrations. Surfactant mixtures are used to formulate simple cleansing formulations, whose performance is also investigated by the developed in vitro approach. The results obtained allow for a good rating of the formulations, which correlates well with the performance of the surfactant mixtures and their interfacial activity.
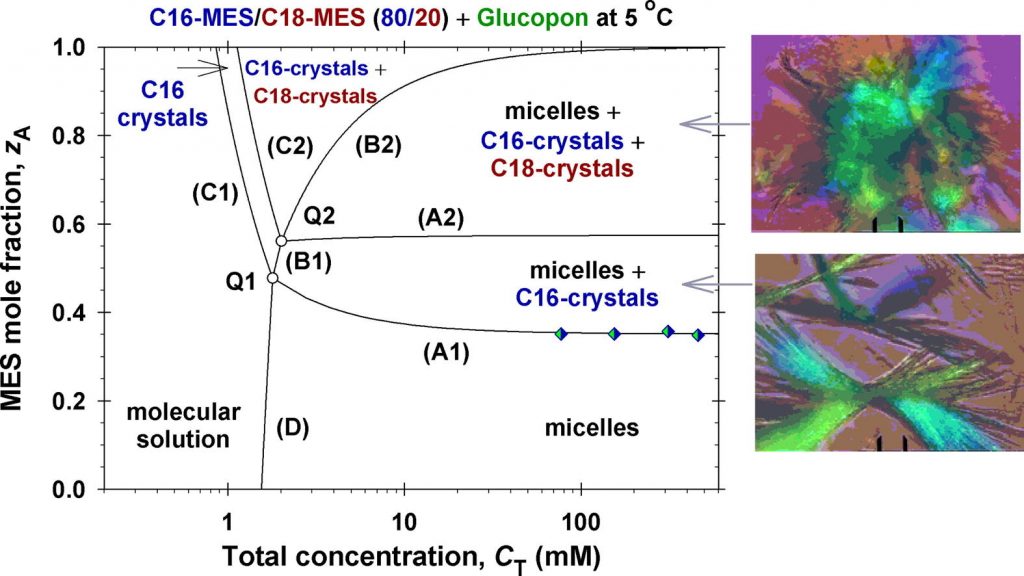
Enhanced solubility of methyl ester sulfonates below their Krafft points in mixed micellar solutions
Hypothesis: Methyl ester sulfonates (MES) show limited water solubility at lower temperatures (Krafft point). One way to increase their solubility below their Krafft points is to incorporate them in anionic surfactant micelles. The electrostatic interactions between the ionic surfactant molecules and charged micelles play an important role for the degree of MES solubility. Experiments: The solubility and electrolytic conductivity for binary and ternary surfactant mixtures of MES with anionic sodium alpha olefin sulfonate (AOS) and sodium lauryl ether sulfate with two ethylene oxide groups (SLES-2EO) at 5 °C during long-term storage were measured. Phase diagrams were established; a general phase separation theoretical model for their explanation was developed and checked experimentally. Findings: The binary and ternary phase diagrams for studied surfactant mixtures include phase domains: mixed micelles; micelles + crystallites; crystallites, and molecular solution. The proposed general phase separation model for ionic surfactant mixtures is convenient for construction of such complex phase diagrams and provides information on the concentrations of all components of the complex solution and on the micellar electrostatic potential. The obtained maximal MES mole fraction of transparent micellar solutions could be of interest to increase the range of applicability of MES–surfactants.
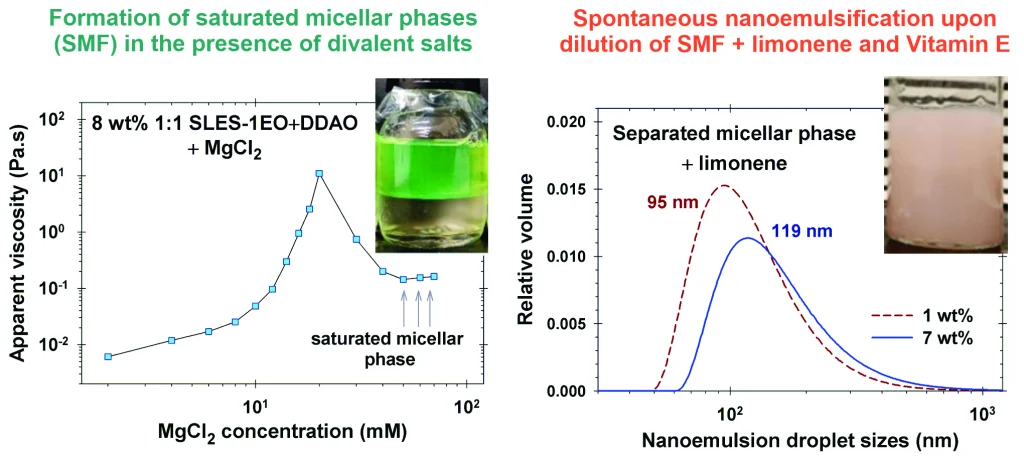
Saturated micellar networks: Phase separation and nanoemulsification capacity
Different oils can be homogeneously dispersed in the network junctions of the separated bicontinuous micellar phases. Upon dilution, these dispersions spontaneously form nanoemulsions. The possibility of a micellar sponge phase formation in the case of mixtures with three anionic and two zwitterionic surfactants in the presence of divalent and monovalent salts is studied. The best results are obtained using sodium lauryl ether sulfate with 1 ethylene oxide group (SLES-1EO) and both cocamidopropyl betaine (CAPB) or N,N-dimethyldodecylamine N-oxide (DDAO) in the presence of an appropriate small amount of MgCl2 and CaCl2. Bicontinuous micellar phases can be produced also in high-salinity NaCl solutions. The bulk properties of these phases are independent of the concentration of the initial solutions from which they are separated, and their Newtonian viscosities are in the range from 0.3 Pa·s to 0.8 Pa·s. Both 8 wt% CAPB- and DDAO-containing sponge phases engulf up to 10 wt% limonene and spontaneously form nanoemulsion upon dilution with droplet sizes of 110–120 nm. Vitamin E can be homogeneously dispersed only in CAPB-containing saturated micellar network, and upon dilution, these dispersions spontaneously form nanoemulsions with smaller droplet sizes of 66 nm for both 8 diastereomers and 2 diastereomers mixtures of vitamin E.
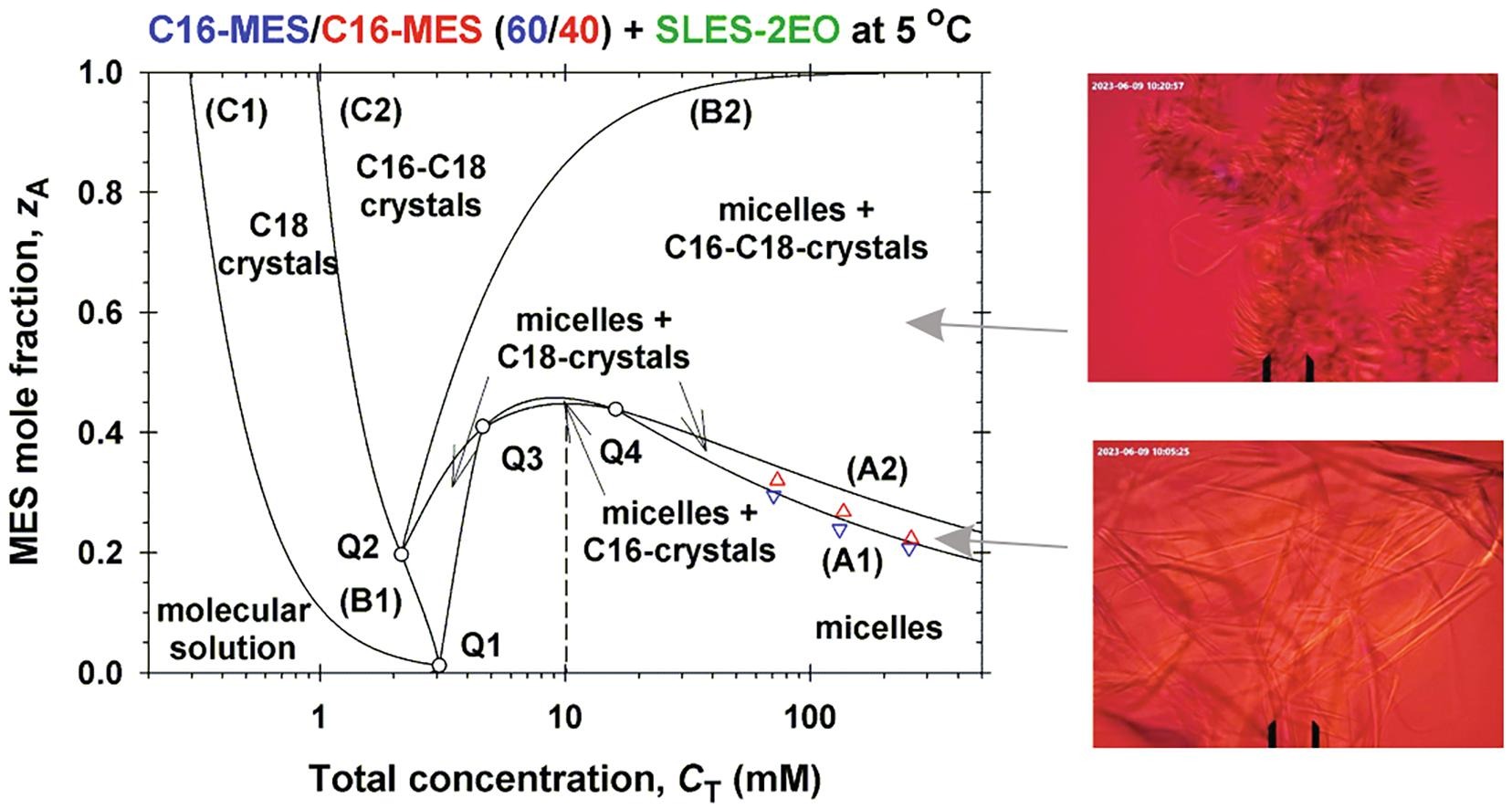
Solubility of ionic surfactants below their Krafft point in mixed micellar solutions: Phase diagrams for methyl ester sulfonates and nonionic cosurfactants
Hypothesis: Many ionic surfactants with wide applications in personal-care and house-hold detergency show limited water solubility at lower temperatures (Krafft point). This drawback can be overcome by using mixed solutions, where the ionic surfactant is incorporated in mixed micelles with another surfactant, which is soluble at lower temperatures. Experiments: The solubility and electrolytic conductivity for a binary surfactant mixture of anionic methyl ester sulfonates (MES) with nonionic alkyl polyglucoside and alkyl polyoxyethylene ether at 5 °C during long-term storage were measured. Phase diagrams were established; a general theoretical model for their explanation was developed and checked experimentally. Findings: The binary and ternary phase diagrams for studied surfactant mixtures include phase domains: mixed micelles; micelles + crystallites; crystallites, and molecular solution. The proposed general methodology, which utilizes the equations of molecular thermodynamics at minimum number of experimental measurements, is convenient for construction of such phase diagrams. The results could increase the range of applicability of MES–surfactants with relatively high Krafft temperature, but with various useful properties such as excellent biodegradability and skin compatibility; stability in hard water; good wetting and cleaning performance.