Assist. Prof. Svetoslav E. Anachkov, Ph.D.
Interests
- Surface Forces in Colloidal Dispersions
- Micellization and Self-Assembly
- Nanoparticle Synthesis and Ordering
Bio
Publications
Most recent publications
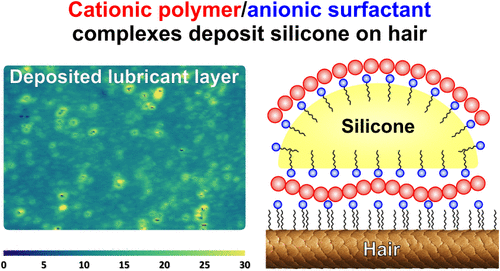
Optimizing lubricant deposition on hair-like substrates using cationic polymer/anionic surfactant complexes
Oppositely charged polymer–surfactant mixtures exhibit unique bulk and interfacial properties with many applications. In particular, cationic polymers paired with anionic surfactants are often used to deposit oils and lubricants on hair and skin upon dilution. These dilution-deposition systems are widely studied in simple mixtures but rarely in complex formulations. Thus, our paper focuses on cationic polysaccharides (cat-Guars and cat-HECs) paired with the anionic surfactant SLES-1EO (sodium laureth-1 sulfate) and incorporated into shampoos. We analyzed the polymer–surfactant complexes’ (PSCs) phase behavior, adsorption at the silicone oil/water interface, stickiness to bubbles (and drops), and deposition on hair-like substrates via phase behavior analysis, zeta potential measurements, foam film experiments, and imaging ellipsometry. Our results showed that the cat-Guar/SLES-1EO complexes exhibit wider precipitation regions and higher adsorption at the silicone oil/water interface than the cat-HEC/SLES-1EO complexes. Foam film experiments implied that only the cat-Guar/SLES-1EO complexes bridge the air bubbles (and silicone drops to hair) as they form sticky PSCs. Imaging ellipsometry revealed that cat-Guars deposit thick, inhomogeneous layers of PSCs and silicone on the hair-like substrates, whereas cat-HECs deposit thinner layers or nothing. Together, these findings elucidate the underlying deposition mechanism and offer strategies to optimize the polymer performance in shampoo formulations via a comprehensive experimental protocol.
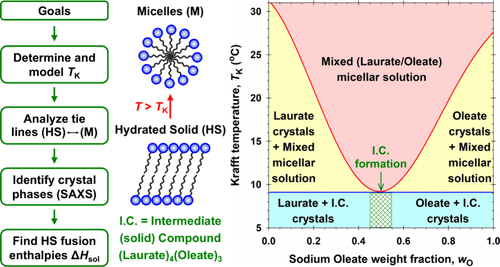
Reexamining the enhanced solubility of sodium laurate/sodium oleate eutectic mixtures
Mixtures of multiple surfactants that have superior performance to the individual components are highly sought-after commercially. Mixtures with a reduced Krafft point (TK) are particularly useful as they enable applications at lower temperatures. Such an example is the soap maker’s eutectic: the mixture of sodium laurate (NaL) and sodium oleate (NaOl). A true eutectic implies that the two surfactants do not mix in the solid state but mix readily in the micellar solution above TK, leading to a sharp TK depression at a specific composition. However, the NaL/NaOl mixture shows a broad TK depression of >15 °C at a NaOl weight fraction (wO) of about 0.5. Our tie-line analysis shows that pure NaL and NaOl do not coexist in the solid phase on either side of the TK minimum. X-ray analysis of the isolated solids with varying wO reveals that a unique intermediate compound (I.C.) forms in the solid state with a NaL-to-NaOl mole ratio of about 4/3. Below the TK minimum, NaL and the I.C. coexist in the solids for wO 0.5. Each pair of solids exhibits eutectic or monotectic solubility behavior, and the congruent I.C. melting point is so close to that of the eutectic point(s) that a broad TK minimum ensues. Thermal analysis and modeling via the freezing-point depression approach support the above interpretation. The fact that surfactants with other headgroups but the same blend of chain lengths do not exhibit similar depressed TK is a topic for further study.
Oral care composition used for whitening teeth, comprises nonionic surfactant comprising one or more carbon-carbon double bond, pigment and carrier, and does not comprise anionic surfactants, amphoteric surfactants and polymers
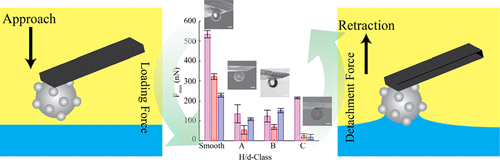
Detachment of rough colloids from liquid–liquid interfaces
Particle surface roughness and chemistry play a pivotal role in the design of new particle-based materials. Although the adsorption of rough particles has been studied in the literature, desorption of such particles remains poorly understood. In this work, we specifically focus on the detachment of rough and chemically modified raspberry-like microparticles from water/oil interfaces using colloidal-probe atomic force microscopy. We observe different contact-line dynamics occurring upon particle detachment (pinning vs sliding), depending on both the particle roughness and surface modification. In general, surface roughness leads to a reduction of the desorption force of hydrophobic particles into the oil and provides a multitude of pinning points that can be accessed by applying different loads. Our results hence suggest future strategies for stabilization and destabilization of Pickering emulsions and foams.
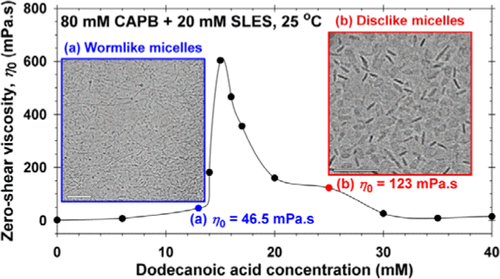
Viscosity peak due to shape transition from wormlike to disklike micelles: Effect of dodecanoic acid
Here, we have investigated the synergistic growth of long wormlike micelles and their transformation into disklike micelles, which occurs in three-component solutions composed of sodium lauryl ether sulfate (SLES; anionic), cocamidopropyl betaine (CAPB; zwitterionic), and dodecanoic acid (HC12; nonionic). The solution rheology is characterized in terms of zero-shear viscosities and characteristic times for micellar breaking and reptation. Furthermore, the microstructure evolution, leading to the observed rheological behavior, is revealed by cryo-transmission electron microscopy (TEM) micrographs. In all cases, the CAPB-to-SLES ratio is fixed, whereas the fatty acid concentration is varied. At a certain HC12 concentration, the solution viscosity passes through a maximum. The cryo-TEM imaging indicates that wormlike micelles appear before the peak, grow further up to the peak, and finally transform into disklike aggregates (a very rare micellar structure) after the peak. The transformation of worms into disks leads to a drop in viscosity because the length-to-thickness aspect ratio of the disks is significantly lower than that of the worms. In this article, we elucidate the structure-rheology relations in micellar solutions that might be applied for the design of personal-care and household formulations.