Prof. Slavka S. Tcholakova, Ph.D.
Head of the Laboratory for Active Formulations and Materials
Interests
- Formation, stability and rheology of foams and emulsions
- Mechanism of action of antifoams
- Surfactant aggregates and mesophases in bulk: structure, rheology, stability
- Biophysics & colloid science in fat digestion and drug delivery systems
- Defoamers and antifoams
- Physicochemical theoretical models for foamability, emulsification; foam rheology
- Natural based surfactants
Bio
Prof. Slavka Tcholakova received M.Sc. in Chemistry (1996), Ph.D. in Physical Chemistry (2004), Assistant Professor (2006), Associate Professor (2009), and became a Full Professor in 2013 in the Faculty of Chemistry and Pharmacy, Sofia University, Bulgaria. She was a head of the Department of Chemical and Pharmaceutical Engineering (DCPE) in Sofia University from 2015 to 2024. Currently, she is a head of the Laboratory “Active formulations and materials” at DCPE. She has been a visiting researcher in the Research Center Paul Pascal, CNRS, Bordeaux, France (1997).
Her research interests include formation and stability of emulsions; rheology of foams and emulsions; protein adsorption in relation to emulsion stability; foam stability in the presence of antifoams; in-vitro models for digestion and bioavailability of hydrophobic molecules and the respective experimental methods. So far, she has published 135 research and review papers, cited over 5 600 times (h-index = 42). She has been leading over 60 project and participating in more than 30 projects with international companies(BASF, Unilever, Saint Gobain, Wacker, Lubrizol, PepsiCo, Altana, BYK Chemie, Productolysa, etc.). She has been a supervisor and co-supervisor of 16 completed PhD Theses. She was the recipient of the “Best Young Scientist” award for 2006 of the Sofia University Foundation “St. Kliment Ohridski”. In 2018, Prof. Tcholakova received the National Bulgarian award “Pythagoras” for high scientific achievements in category Natural Sciences. Based on their publications and citations received in the past year only, Prof. Tcholakova has been ranked 1712 in Chemical Physics category among 106 831 scientists included in the Stanford/Elsevier’s Top 2% Scientists Ranking in 2024.
Publications
Most recent publications
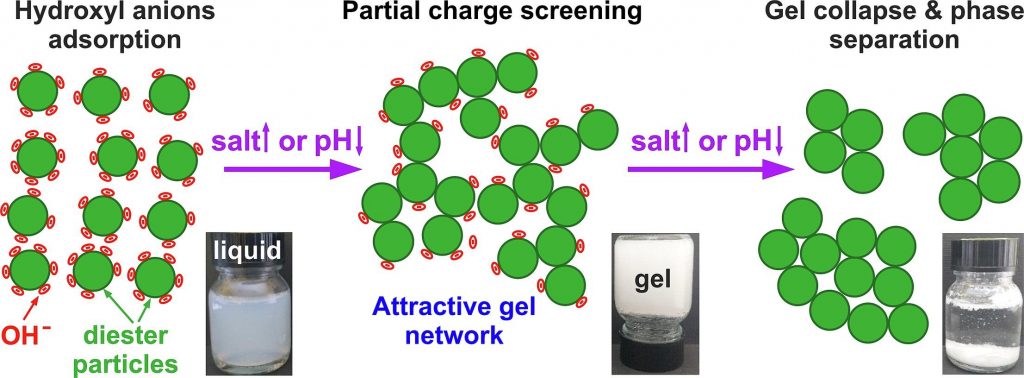
Salt-induced gelation of nonionic sucrose ester dispersions
Hypothesis
The dispersions of nonionic sucrose ester surfactants in water exhibit a highly negative zeta-potential, though its origin remains controversial. The addition of electrolytes to these dispersions may influence their zeta-potential, thus potentially affecting their physicochemical properties.
Experiments
The electrolyte- and pH- driven gelation of aqueous dispersions of commercial sucrose stearate (S970) containing ca. 1:1 monoesters and diesters was studied using optical microscopy, rheological and zeta-potential measurements, and small-angle X-ray scattering techniques.
Findings
At low electrolyte concentrations and pH ≳ 5, 0.5–5 wt% S970 dispersions exhibited low viscosities and behaved as freely flowing liquids. The addition of electrolytes of low concentrations, e.g. 9 mM NaCl or 1.5 mM MgCl2, induced the formation of a non-flowing gels. This sol–gel transition occurred due to the partial screening of the diesters particles charge, allowing the formation of an attractive gel network, spanning across the dispersion volume. Complete charge screening, however, led to a gel-sol transition and phase separation. Gel formation was observed also by pH variation without electrolyte addition, whereas the addition of free fatty acids had negligible impact on dispersion properties. These findings support the hypothesis that the negative charge in sucrose ester dispersions arises from hydroxyl anions adsorption on particles surfaces. Gels were formed using just 1.3 wt% surfactant, and the critical electrolyte concentration for gelation was found to scale approximately with the square of the cation charge, in agreement with the low surface charge density theory. The biodegradable sucrose esters gels offer a sustainable alternative for structuring personal and home care products, replacing the wormlike micelles of synthetic surfactants typically used at much higher surfactant and salt concentrations.
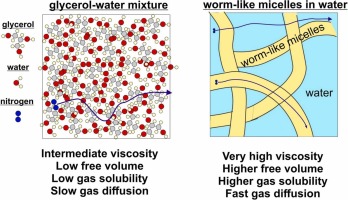
Role of dispersion nanostructure for bubble dissolution under pressure
Understanding the factors that affect bubble dissolution under pressure is crucial for the pneumatic transport of dispersions. This study probes the kinetics of air dissolution, the air solubility at a given pressure, and the gas diffusion due to bubble dissolution to elucidate the molecular mechanisms of gas transport in liquid dispersions with varied structures and viscosities. We achieve our aims by using water-glycerol mixtures, silicone oils with different viscosities, surfactant solutions containing worm-like micelles and having different macroscopic viscosities, particle suspensions, and surfactant solutions capable of forming a condensed adsorption layer on the bubble surfaces at ambient conditions. The results show that the dissolution rate does not depend on the macroscopic viscosity for silicone oils and solutions containing worm-like micelles, indicating that gas diffusion occurs faster than the movement of big polymeric molecules and worm-like micelles. We could predict the experimentally determined diffusion coefficients by accounting for free volume in these media and using the equation for Knudsen diffusion. We show that one way to decrease the rate of bubble dissolution under pressure is to add surfactants, which can decrease the permeability of the adsorption layer formed on the bubble surface by forming a condensed adsorption layer.
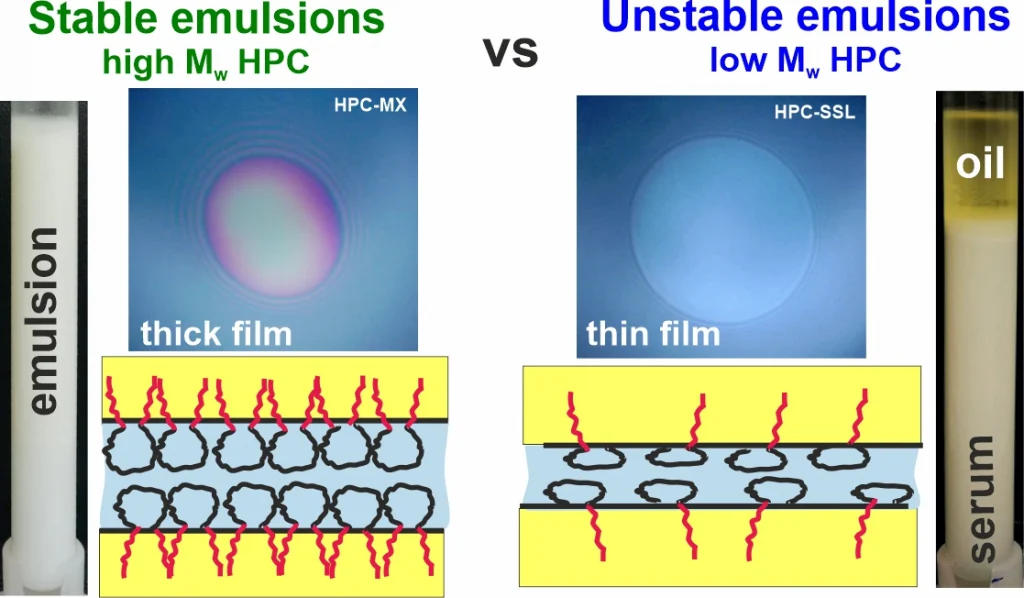
Hydroxypropyl cellulose polymers as efficient emulsion stabilizers: The effect of molecular weight and overlap concentration
Hydroxypropyl cellulose (HPC) is a non-digestible water-soluble polysaccharide used in various food, cosmetic, and pharmaceutical applications. In the current study, the aqueous solutions of six HPC grades, with molecular mass ranging from 40 to 870 kDa, were characterized with respect to their precipitation temperatures, interfacial tensions (IFTs), rheological properties and emulsifying and stabilization ability in palm (PO) and sunflower (SFO) oil emulsions. The main conclusions from the obtained results are as follows: (1) Emulsion drop size follows a master curve as a function of HPC concentration for all studied polymers, indicating that polymer molecular mass and solution viscosity have a secondary effect, while the primary effect is the fraction of surface-active molecules, estimated to be around 1–2% for all polymers. (2) Stable emulsions were obtained only with HPC polymers with Mw ≥ 400 kDa at concentrations approximately 3.5 times higher than the critical overlap concentration, c*. At PO concentrations beyond 40 wt. % or when the temperature was 25 °C, these emulsions appeared as highly viscous liquids or non-flowing gels. (3) HPC polymers with Mw < 90 kDa were unable to form stable emulsions, as the surface-active molecules cannot provide steric stabilization even at c ≳ 4–5 c*, resulting in drop creaming and coalescence during storage.
Rheology of dispersions containing non-spherical lipid particles
The rheological properties of disperse systems play a crucial role in the production of foods, cosmetics, and pharmaceuticals with desired characteristics. Emulsion viscosity can be increased through various methods, incl. increasing the oil volume fraction, incorporating rheological modifiers, or inducing partial coalescence between the droplets. It is well known that suspensions containing inorganic non-spherical particles often exhibit significantly higher viscosities when compared to those with spherical particles. The spontaneous drop self-shaping phenomenon in emulsions, first reported in detail by Denkov et al. (Nature, 2015, 528, 392–395), enables the formation of fluid and frozen lipid particles with regular non-spherical shapes, including platelets, rods and fibers. In this study, we utilize this approach to prepare emulsions containing non-spherical frozen particles of various shapes and investigate their rheological properties. The effects of oil volume fraction, surfactant type, initial drop size and polydispersity are investigated. The results reveal that non-flowing, gel-like samples can be prepared at ca. 11 vol% oil fraction when the emulsion contains polydisperse droplets which acquire non-spherical shapes upon cooling. For comparison, more than ca. 65 vol% oil is needed to obtain similar rheological characteristics in samples containing spherical particles. Additionally, we demonstrate that the optimal drop size for gel preparation is d32 ≈ 4–13 μm. The obtained results are explained mechanistically, and guiding principles are provided for preparing emulsions with increased viscosities using this new approach.

ISCOM-type matrix from beta-escin and glycyrrhizin saponins
Background and aims
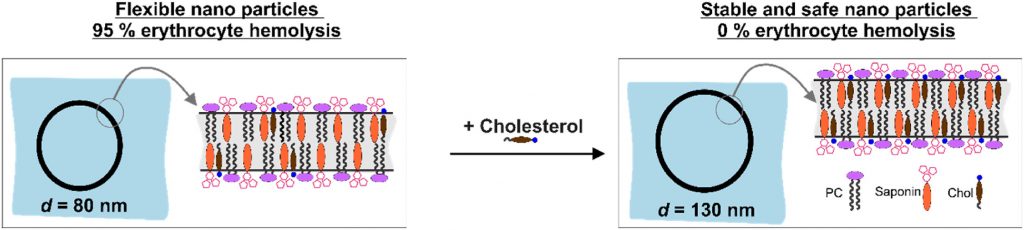
Nanotechnology provides the opportunity for construction of modern transport devices such as nanoparticles for a variety of applications in the field of medicine. A novel experimental protocol for the formation of saponin-cholesterol-phospholipid nanoparticles of vesicular structure has been developed and applied to prepare stable nanoparticles using escin or glycyrrhizin as saponins.
Methods
The methods for nanoparticle construction include a sonication at 90 °C of the initial mixture of components, followed by an additional sonication on the next day for incorporation of an additional amount of cholesterol, thus forming stable unilamellar vesicles. Tests and assays for cell viability, erythrocyte hemolysis, flow cytometry, and fluorescent microscopy analyses have been performed.
Results
By selecting appropriate component ratios, stable and safe particles were formulated with respect to the tested bio-cells. The prepared nanoparticles have mean diameter between 70 and 130 nm, depending on their composition. The versatility of these nanoparticles allows for the encapsulation of various molecules, either within the vesicle interior for water-soluble components or within the vesicle walls for hydrophobic components. The saponin particles formed after cholesterol post-addition (E3-M2) are stable and 100 % of the cells remain viable even after 10-times dilution of the initial particle suspension. These particles are successful included into isolated mouse macrophages.
Conclusions
Among the variety of generated nanoparticles, the E3-M2 particles demonstrated properties of safe and efficient devices for future vaccine design and antigen targeting to immune system.