Prof. Slavka S. Tcholakova, Ph.D.
Head of the Laboratory for Active Formulations and Materials
Interests
- Formation, stability and rheology of foams and emulsions
- Mechanism of action of antifoams
- Surfactant aggregates and mesophases in bulk: structure, rheology, stability
- Biophysics & colloid science in fat digestion and drug delivery systems
- Defoamers and antifoams
- Physicochemical theoretical models for foamability, emulsification; foam rheology
- Natural based surfactants
Bio
Prof. Slavka Tcholakova received M.Sc. in Chemistry (1996), Ph.D. in Physical Chemistry (2004), Assistant Professor (2006), Associate Professor (2009), and became a Full Professor in 2013 in the Faculty of Chemistry and Pharmacy, Sofia University, Bulgaria. She was a head of the Department of Chemical and Pharmaceutical Engineering (DCPE) in Sofia University from 2015 to 2024. Currently, she is a head of the Laboratory “Active formulations and materials” at DCPE. She has been a visiting researcher in the Research Center Paul Pascal, CNRS, Bordeaux, France (1997).
Her research interests include formation and stability of emulsions; rheology of foams and emulsions; protein adsorption in relation to emulsion stability; foam stability in the presence of antifoams; in-vitro models for digestion and bioavailability of hydrophobic molecules and the respective experimental methods. So far, she has published 150 research and review papers, cited over 6300 times (h-index = 44). She has been leading over 70 projects and participating in more than 30 projects with international companies (BASF, Unilever, Saint Gobain, Wacker, Lubrizol, PepsiCo, Altana, BYK Chemie, Productolysa, etc.).
She has been a supervisor and co-supervisor of 16 completed PhD Theses. She was the recipient of the “Best Young Scientist” award for 2006 of the Sofia University Foundation “St. Kliment Ohridski”. In 2018, Prof. Tcholakova received the National Bulgarian award “Pythagoras” for high scientific achievements in category Natural Sciences.
Based on her publications and citations in 2023 and 2024, Prof. Tcholakova achieved high rankings in the Chemical Physics category of the Stanford/Elsevier’s Top 2% Scientists Ranking: In 2024, she was ranked 1712 out of 106,831 scientists; In 2025, she was ranked 1764 out of 115,551 scientists.
Publications
Most recent publications
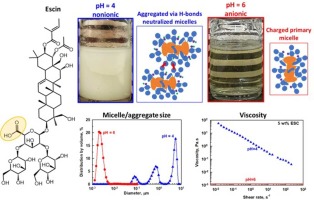
Escin solutions: Effects of pH and electrolytes on their behavior
Escin is a triterpenoid saponin with one carboxyl group which is non-ionized at pH 4.7. The major aim of the current study is to determine how the electrolyte concentration affects the properties of concentrated escin solutions (5 wt% and 10 wt%) at pHs of 4, 6, and 8. Ionized escin molecules at pH >4.7 form charged micelles that repel one another when there is no added electrolyte and solutions remain clear and stable for more than a month. Lowering the pH to 4 leads to formation of uncharged micelles. These micelles attract each other and form inter-micellar hydrogen bonds, which enable formation of micrometer aggregates that cause turbidity and phase separation. The addition of background electrolytes to the solutions at pHs of 6 and 8 screens the electrostatic repulsion between micelles, causing partial aggregation of the micelles and gelation of solutions. As the salt concentration increases, the viscosity of the escin solution also increases, reaching a maximum—similar to the behavior observed with conventional surfactants. However, the mechanism behind this viscosity maximum is different. In solutions of conventional surfactants, the maximum is due to the formation of worm-like micelles, whereas the maximum for escin solutions is due to formation of a network of escin aggregates that imparts yield stress and elasticity to the solution. These dispersions remain stable for at least one month at room temperature and can be used as cosmetic and detergent formulations.
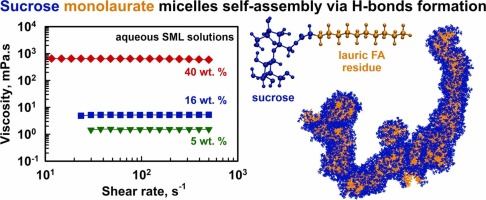
Sucrose monolaurate self-assembly via hydrogen bonding: role of surfactant concentration and urea
Sugar esters, a class of surfactants derived from renewable resources, have attracted significant attention due to their biodegradability, low toxicity, and broad applications in food, cosmetic, and pharmaceutical formulations. Despite their widespread use, the phase behavior of these compounds in aqueous systems remains incompletely understood. In this study, we investigate the self-assembly of a nonionic sucrose ester of lauric acid in 1–40 wt% concentration range using rheological measurements, dynamic light scattering, X-ray scattering, DOSY NMR, and molecular dynamics simulations. Formation of spherical micelles with a diameter of 5.4 nm is observed at low surfactant concentrations, driven by hydrophobic interactions between the alkyl tails. These solutions exhibit Newtonian flow behavior with viscosities close to that of pure water. However, the viscosity increases from 5 mPa.s at 16 wt% to 640 mPa.s at 40 wt%, while the Newtonian character persists even at 40 wt%. This behavior is explained with the formation of interconnected, thread-like micellar structures of (almost) spherical micelles that largely preserve their distinctiveness, resembling the “pearl necklace” arrangement known for polymer systems. The main driving force for this supramolecular organization was found to be the hydrogen bonding between sucrose headgroups. The addition of 6 M urea, a known hydrogen bond disruptor, significantly reduces micelle clustering and the viscosity decreases to 150 mPa.s at 40 wt% concentration, supporting the proposed aggregation mechanism. These findings contribute to a deeper understanding of the self-assembly behavior of sucrose esters in aqueous environment and highlight their potential for controlled aggregation in practical formulations.
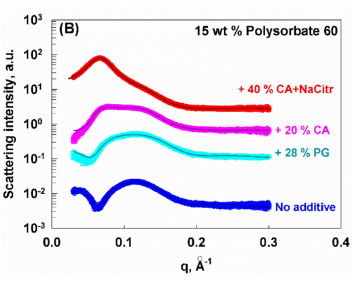
Role of food additives on properties of Polysorbate 60 solutions
Polysorbates are hydrophilic, nonionic surfactants widely used in food, cosmetic, and pharmaceutical products. Often, these emulsifiers are used in combination with different preservatives, and it is essential to conduct an in-depth study of the influence of such additives on the properties of Polysorbate 60 solutions. In the current study, we investigated the effect of various food additives: citric acid, sodium benzoate, potassium sorbate, a mixture of citric acid and sodium citrate, and propylene glycol on the stability and micellar properties of Polysorbate 60 by using different experimental methods such as GC, DSC, SAXS, DLS, optical observations in polarised light, and NMR. When stored at room temperature without additives, solutions of Polysorbate 60 slowly undergo phase separation over time. Our results show that citric acid, a mixture of citric acid and sodium citrate, and propylene glycol increase the rate of this phase separation. In contrast, sodium benzoate and potassium sorbate are incorporated into the mixed micelles. They localize within the palisade layer, a region of the micelle where the surfactant tails begin, effectively preventing the phase separation. As a result, Polysorbate 60 solutions with these specific additives remain transparent and stable for over a year
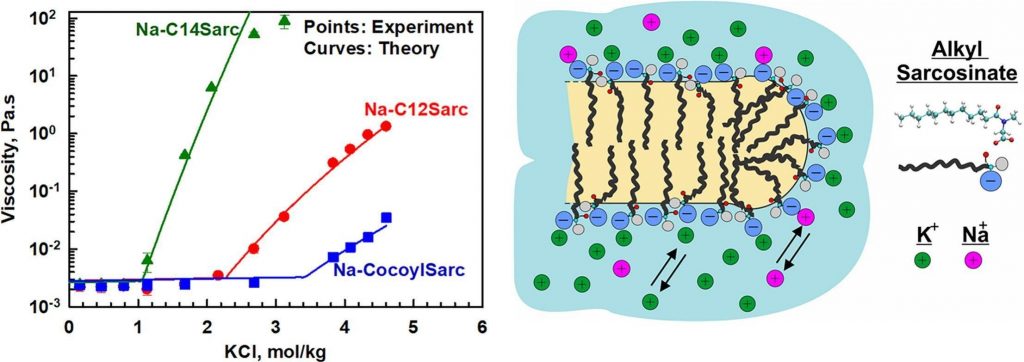
Role of electrolytes and co-surfactants on the rheological properties of sodium N-acyl sarcosinate solutions
Alkyl sarcosinates are amino acid-based anionic surfactants commonly used as primary surfactants in sulfate-free personal care products. The major aim of this study is to identify the key factors influencing the rheological behaviour of sodium sarcosinate solutions and their mixtures with nonionic, zwitterionic, and cationic co-surfactants. To achieve this, we examined the effects of salt type and concentration for alkyl sarosinates with different chain lengths (dodecyl, tetradecyl, and cocoyl) across concentration range of 2–20 wt%. Experimental results reveal two distinct regions in the salt curve for the three studied sarcosinates. At low electrolyte concentrations, viscosity remains constant until reaching the critical electrolyte concentration, C1, beyond which viscosity increases logarithmically with salt concentration. Further electrolyte addition leads to phase separated solutions at the critical precipitation concentration, CTR. Both C1 and CTR decrease as the hydrocarbon chain length increases from dodecyl to tetradecyl. However, the presence of shorter chain molecules in cocoyl sarocisinate significantly increases both C1 and CTR due to the formation of spherical micelles. A theoretical expression for predicting viscosity dependence on salt concentration is derived and successfully applied to describe the experimental data. The adsorption energy of sodium and potassium to alkyl sacrosinate micelle surfaces is found to be much smaller than that to sodium lauroyl ether sulfate surfactants (1 vs. 3 kBT for Na+ and 0.8 vs. 3.8 kBT for K+). No significant effect of amphoteric co-surfactants, including cocoamidopropyl betaine, sulfobetaine, or decylamine oxide, is observed. NMR analysis confirms that cocoamidopropyl betaine and sodium dodecyl sarcosinate form mixed micelles that are structurally similar to sarcosinate micelles, as carboxyl groups remain exposed on the micelle surfaces in both cases. When using amine oxide and sulfobetaine, the increase in viscosity is attributed to the elongation of mixed micelles, though steric hindrance from side methyl groups limits their growth. The practical significance of this study lies in the finding that longer-chain alkyl sarcosinates (such as tetradecyl, as investigated here) can attain significantly higher viscosities at lower salt concentrations compared to shorter-chain analogs or surfactant mixtures. The scientific significance stems from the development of a theoretical model capable of predicting the viscosity of alkyl sarcosinate solutions across various surfactant and salt concentrations.
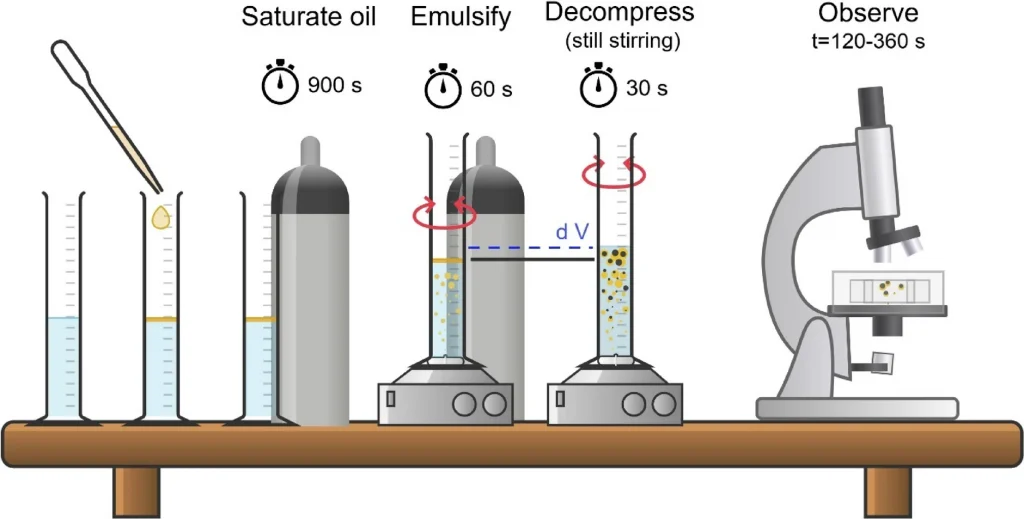
Bubbles nucleation in supersaturated emulsion drops
Bubble nucleation plays a significant role in applications ranging from food and beverages to cosmetics, polymer foams, and advanced porous materials. While extensively studied in homogeneous solutions and particle suspensions, bubble nucleation mechanisms in heterophasic liquid dispersion, such as emulsions, are less understood. This study hypothesizes that tuning physicochemical and mixing hydrodynamics allows design over the bubble nucleation and growth under mild gas supersaturations. Supersaturated oils were emulsified under mild stirring, followed by rapid decompression to trigger nucleation. The process was analyzed by monitoring changes in emulsion volume and optical microscope observations. Key parameters such as gas saturation pressure, viscosities of the continuous and dispersed phases, gas solubility and dissolution kinetics, and mixing intensity were systematically varied. Bubble nucleation occurs mainly via a heterogeneous mechanism, accelerated by shear and gas migration kinetics. Increased oil phase viscosity enhanced bubble formation and retention in droplets, while higher aqueous phase viscosity suppressed nucleation in the continuous phase. The number and size of the obtained bubbles varied significantly, depending on the phase of nucleation origin and the physicochemical conditions. This study reveals pathways to optimize bubble nucleation and initial growth dynamics, which can be used for optimization of pore size distribution of emulsion-based materials.