Rumyana D. Stanimirova, Ph.D.
Interests
- Experimental methods for measuring of dynamic and equilibrium surface tension
- Adsorption of proteins and surfactants on fluid and solid interfaces
- Kinetics of Surfactant Adsorption
- Surface rheology of fluid interfaces
- Contact angles and wetting on solid surfaces
- Atomic force microscopy (AFM)
- Cleaning of skin, solid surfaces and fabrics
Bio
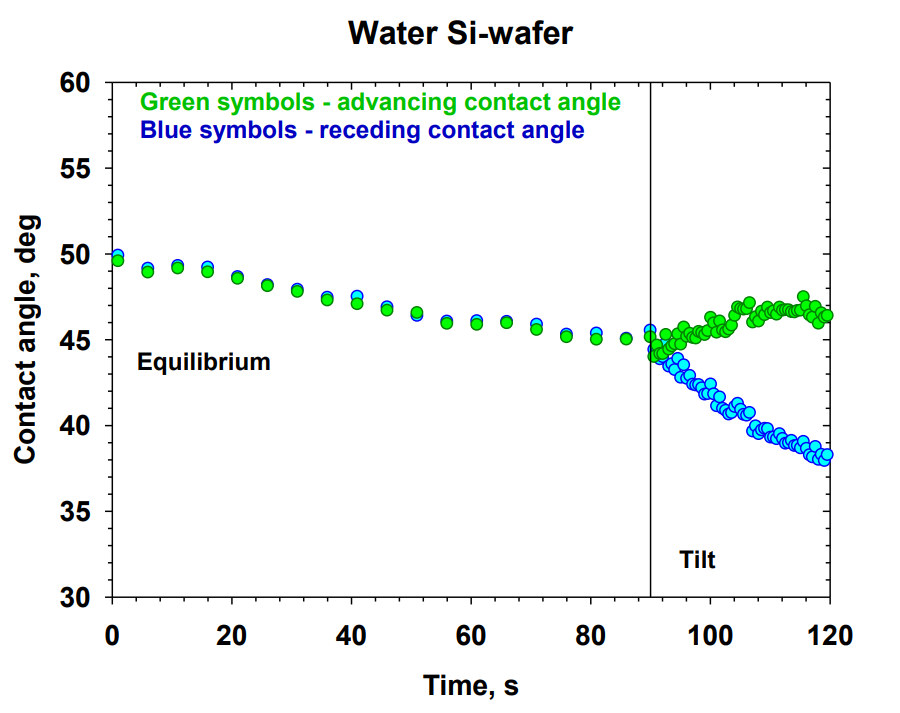
Rumyana Stanimirova was born on 4-th September 1983 in Haskovo, Bulgaria. In 2002 she graduated the High school of science and mathematics “Boyan Petkanchin” in Haskovo, Bulgaria. In 2006 she got the B.Sc degree in Chemistry, Faculty of Chemistry of Sofia University “St. Kliment Ohridski”. In 2009 she received M.Sc.degree in “Colloidal systems in modern science and technology”, Faculty of Chemistry of Sofia University “St. Kliment Ohridski”. In 2014 she got the PhD in Faculty of Chemistry and Pharmacy of Sofia University “St. Kliment Ohridski”. Her thesis was “Rheology of Mixed Adsorption Layers of Proteins and Surfactants at Liquid Interfaces” with scientific supervisors Prof. Krassimir Danov Ph.D., D.Sc., and Assoc. Prof. Krastanka Marinova Ph.D. From 2007 until 2014 she worked as research associate in the Department of chemical and pharmaceutical engineering (DCPE), Faculty of Chemistry and Pharmacy of Sofia University “St. Kliment Ohridski”. She has been a visiting researcher in the Research and Development Center in KLK-Oleo, Kuala Lumpur, Malaysia (2015). She is currently on a Post Doc position in the DCPE, Faculty of Chemistry and Pharmacy of Sofia University. Since 2010 she has been involved in teaching course “Separation Processes in Disperse Systems”, “Disperse systems and cosmetic formulation” – exercises. Her research interests includes: experimental methods for measuring of dynamic and equilibrium surface tension; adsorption of proteins and surfactants on fluid and solid interfaces; kinetics of surfactant adsorption and surface rheology of fluid interfaces; contact angles and wetting on solid surfaces; oil drop detachment and deposition on solid surfaces; cleaning of skin, solid surfaces and fabrics. Up to 2023 year she has published 18 articles, cited more than 300 times in the scientific literature (h-index = 9).
Publications
Most recent publications
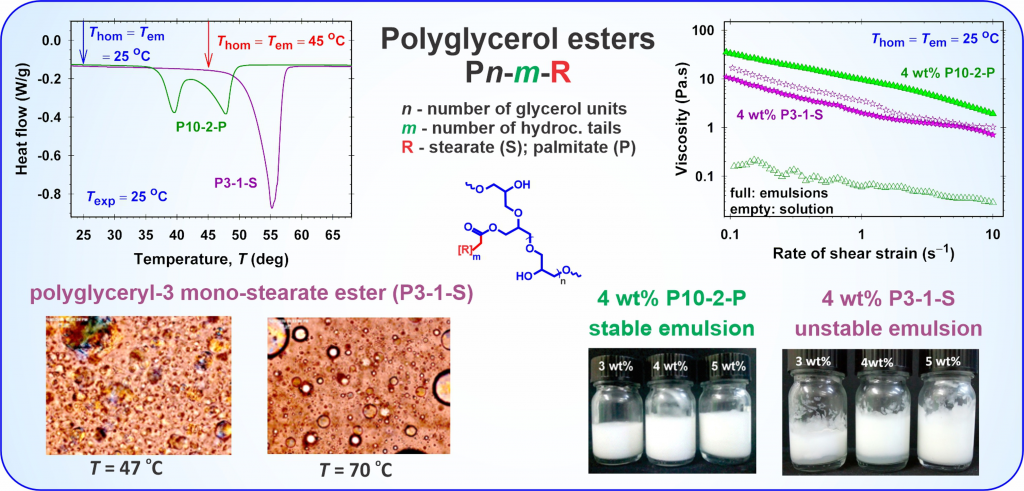
Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities
The polyglycerol esters (PGEs) of fatty acids have a wide range of HLB values and applications in diverse industries, such as pharmaceuticals and cosmetics. While the physicochemical properties of oil-soluble PGEs dissolved in oil phases are well studied in the literature, there is no information on their structuring in aqueous phases and emulsifying abilities. We combined rheological and differential scanning calorimetry (DSC) measurements and microscopy observations to characterize the dependence of oil-soluble PGE structuring in aqueous phases on the PGE concentration, the temperature of solution homogenization, and the PGE molecular structure. Excellent correlations between the considerable changes in solution viscosity and the temperatures of the two endo- and exothermic peaks in the DSC thermograms are observed. Single-tail PGE molecules, which have a higher number of polyglycerol units, are better organized in networks, and the viscosity of their aqueous solutions is higher compared to that of the respective double-tail PGE molecules. PGEs exhibit good emulsifying ability and the viscosity of the produced emulsions at room temperature can differ by orders of magnitudes depending on the temperature of emulsification. The reported properties of oil-soluble PGEs could be of interest for increasing the range of their applicability in practice.
Disperse systems and rheology in clean technologies – lab 6.2 capabilities and developments
Laboratory 6.2 “Disperse systems and rheology in clean technologies” within WP 6 “Nano-structured materials and disperse systems in clean technologies” aims at the development of new systems for clean technologies and nanotechnologies in various scientific and technological areas: new cleaning agents for hard surfaces; encapsulation and controlled release of reagents; wetting and dewetting processes. The main objectives are to conduct experimental and theoretical research and development of new dispersed systems through the inclusion of new raw materials and innovative materials, and to develop and apply theoretical models of the interactions and stability of complex dispersed systems. High scientific output of Lab 6.2 is manifested via 21 scientific publications in the fields of Dispersion Chemistry, Chemical and Materials Sciences for the period 2018 – 2024 (8 articles in Q1 journals, 6 in Q2, and 7 materials in Q4). Optimized compositions of cleaning formulations for hard surfaces with sulfonated methyl esters have been obtained [1]. New theoretical models for the interactions that determine the structures in micellar systems have been developed and applied [2]. New phenomena such as vortexing [3] and nanoemulsification [4] have been described. New methods have been proposed for obtaining emulsions and foams from triglyceride phases [5]. The role of the components and compositions for the rheology [6], wetting, and cleaning [7] has been demonstrated.
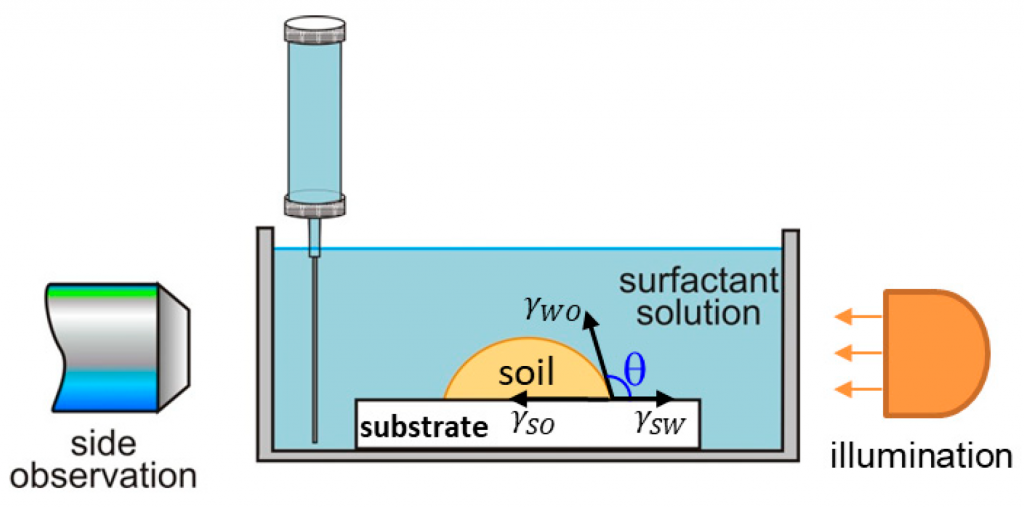
Cleansing mechanisms and efficacy on artificial skin
A systematic study on the mechanisms of cleansing artificial skin by solutions of widely used in personal care surfactants disodium laureth sulfosuccinate (DSLSS), sodium laureth sulfate (SLES), sodium dodecyl sulfate (SDS), dodecyl trimethyl ammonium bromide (DTAB), and coco glucoside (CG), is presented. The systematic characterization of soil removal from artificial skin revealed two primary cleansing mechanisms: emulsification and roll-up. Emulsification occurs in systems with very low interfacial tension, such as sebum in SLES solutions, while dimethicone soil was only removed by roll-up. The roll-up effectiveness depends on the surfactant’s interfacial activity and its adsorption on the soiled surface. Thus, the strong adsorption of DTAB on the skin leads to dimethicone roll-up at a relatively high interfacial tension of 11 mN/m. The anionic and nonionic surfactants adsorbed less at the artificial skin surface, and the oil/water interfacial tension value lowering below 5 mN/m is necessary for the roll-up to occur. Nonionic CG removed dimethicone at a lower concentration than ionic surfactants. Combining CG with ionic surfactants improved cleaning at lower total concentrations. Surfactant mixtures are used to formulate simple cleansing formulations, whose performance is also investigated by the developed in vitro approach. The results obtained allow for a good rating of the formulations, which correlates well with the performance of the surfactant mixtures and their interfacial activity.
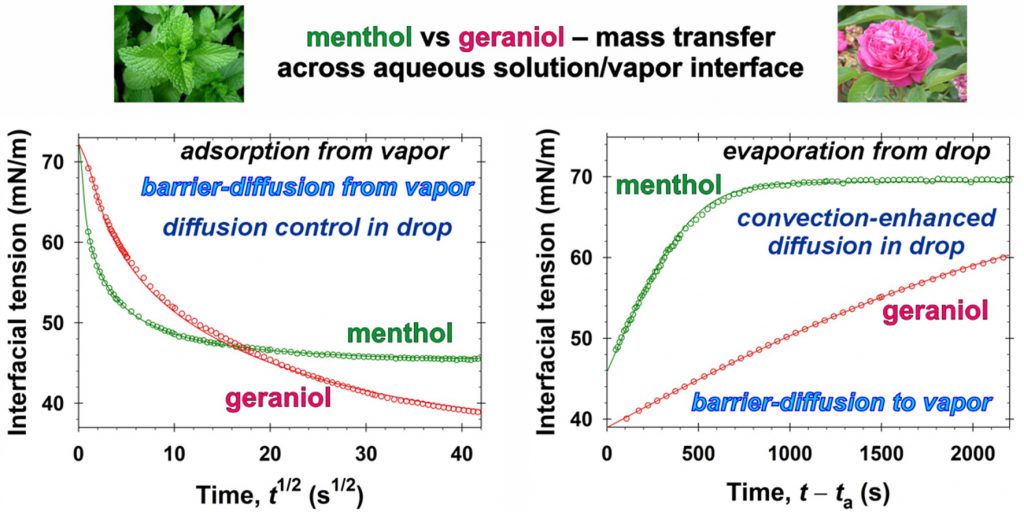
Quantitative characterization of the mass transfer of volatile amphiphiles between vapor and aqueous phases: Experiment vs theory
The class of volatiles, which possess low saturated vapor pressures, appreciable solubilities in water, and well pronounced surface activities, have gained wide applications in diverse areas of industry, cosmetics, and medicine. One way to qualitatively characterize their mass transfer between vapor and aqueous solutions is to measure the relaxation of the interfacial tension, σ, with time, t, under different nonequilibrium initial conditions. This approach is applied in the present work for geraniol and menthol. By means of combining σ(t) data with the respective equilibrium surface tension isotherms, the instantaneous values of the fragrance adsorption, Γ(t), have been determined. Quantitative characterization of the geraniol and menthol mass transfers in the case of adsorption from vapor to aqueous drops is achieved by using a mixed barrier-diffusion model. The obtained values of the rates of adsorption and desorption are compared with those reported in the literature for benzyl acetate, linalool, and citronellol. In the case of evaporation of the volatiles from their saturated aqueous solutions to the ambient atmosphere, the mass transfer is found to be driven both by mixed barrier-diffusion and by convection-enhanced mechanisms – depending on the air humidity. The quantitative description of the evaporation of volatile molecules is modelled theoretically by adsorption rate constants. In order to achieve the reported model representations, complex numerical calculations are implemented. On the other hand, having in mind the cases when one wishes to avoid extensive computational work, we developed a simple semiempirical model suitable for all five studied fragrances. This simplified approach is convenient for the express comparison and characterization of the evaporation rates. The obtained physicochemical parameters related to the evaporation and condensation of volatiles are important for the rigorous modeling of their complex mixed solutions of practical interest. The semiempirical model could be used for the quantitative classification of volatile molecules with respect to their ability to evaporate.
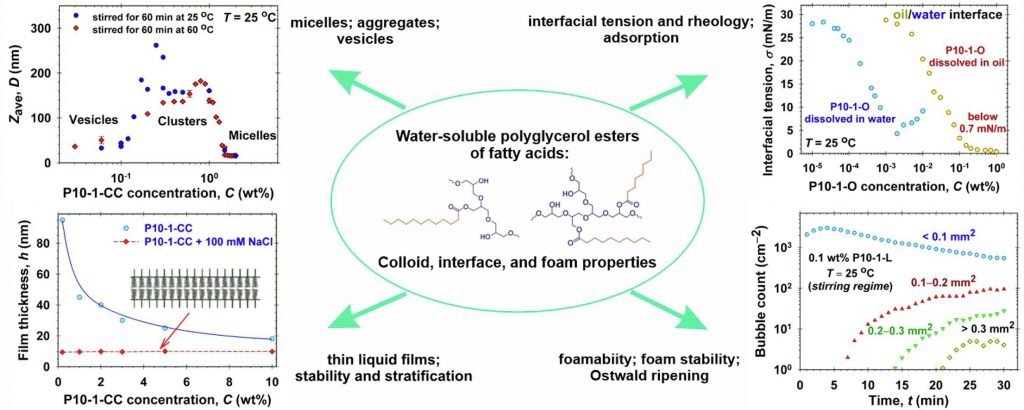
Colloid, interface, and foam properties of water-soluble polyglycerol esters solutions
Hypothesis
Polyglycerol esters of fatty acids are generated via the esterification of a polydisperse mixture of polyglycerol with naturally derived fatty acids. The polymerization process of polyglycerol results in the production of various oligomers, ranging from di-, tri-, and higher-order forms, which contribute to the complexity of final products. The combination of complementary experimental techniques and adequate theoretical interpretations can reveal the wide variety of their physicochemical properties.
Experiments
The colloid and interface properties of polyglyceryl mono-laurate, mono-stearate, mono-oleate, and a mixture of mono-caprylate and mono-caprate esters solutions were characterized by measurements of the electrolytic conductivity, static and dynamic surface tension, aggregate and micelle sizes and distributions, thin liquid film stability and stratification, and solubility in aqueous and in oil phases. The formation, stability, and bubble size distribution of foams generated from polyglycerol esters aqueous solutions were systematically investigated.
Findings
The low concentrations of double-tail molecules and fatty acids in polyglycerol esters affect considerably their micellar, aggregation, and vesicle formations in aqueous solutions. The theoretical data interpretation of polyglycerol esters isotherms and thin liquid films data provide information on the adsorption energies, excluded areas per molecule, interaction parameters of molecules at interfaces, surface electrostatic potential, and the size of micelles. Polyglyceryl mono-oleate exhibits spontaneous emulsification properties. Short chain length polyglycerol esters have excellent foaming ability but relatively low foam stability. The optimal weight fractions of the short-chain polyglyceryl esters and polyglyceryl mono-stearate mixtures with respect to good foaminess and foam stability upon Ostwald ripening are obtained. The reported physicochemical characterization of the water-soluble polyglycerol esters could be of interest to increase the range of their applicability in practice.