Petar Borisov, Ph.D. Student
Interests
- Rheology
- Saponin solutions
Publications
Most recent publications
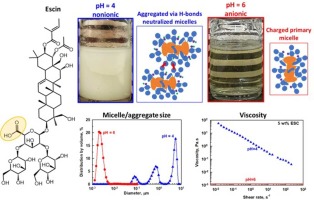
Escin solutions: Effects of pH and electrolytes on their behavior
Escin is a triterpenoid saponin with one carboxyl group which is non-ionized at pH 4.7. The major aim of the current study is to determine how the electrolyte concentration affects the properties of concentrated escin solutions (5 wt% and 10 wt%) at pHs of 4, 6, and 8. Ionized escin molecules at pH >4.7 form charged micelles that repel one another when there is no added electrolyte and solutions remain clear and stable for more than a month. Lowering the pH to 4 leads to formation of uncharged micelles. These micelles attract each other and form inter-micellar hydrogen bonds, which enable formation of micrometer aggregates that cause turbidity and phase separation. The addition of background electrolytes to the solutions at pHs of 6 and 8 screens the electrostatic repulsion between micelles, causing partial aggregation of the micelles and gelation of solutions. As the salt concentration increases, the viscosity of the escin solution also increases, reaching a maximum—similar to the behavior observed with conventional surfactants. However, the mechanism behind this viscosity maximum is different. In solutions of conventional surfactants, the maximum is due to the formation of worm-like micelles, whereas the maximum for escin solutions is due to formation of a network of escin aggregates that imparts yield stress and elasticity to the solution. These dispersions remain stable for at least one month at room temperature and can be used as cosmetic and detergent formulations.