Prof. Nikolai D. Denkov, Ph.D., D.Sc.
Fellow of the Bulgarian Academy of Sciences
Interests
- Foams, Antifoams and Detergency
- Rheology of Foams and Emulsions
- Food Emulsions and Emulsification
- Colloid Crystals and Nanomaterials
- Light Scattering and Electrokinetic Phenomena
Bio
Nikolai D. Denkov received Ph.D. (1993), D.Sc. (2007) and became a Full Professor of Physical Chemistry in 2008. Prof. Denkov served as a vice dean of the Faculty (2004-2008), Head of DCPE department (2008-2015), Deputy Minister (2014-2016) and Minister (2017, 2021-2022) of Education and Science in Bulgaria, and Prime Minister of Bulgaria (2023-2024). He worked as visiting researcher in JRDC (Japan), senior researcher in Rhone-Poulenc R&D (France), lead scientist in Unilever R&D (USA), and guest professor in France (ESPCI-Paris and University of Lille).
His research includes experimental and theoretical studies on the formation, stability, rheology, and applications of disperse systems, and on the surfactant control of their properties. He has published over 190 research articles, including 2 papers in Nature, 1 in Nature Physics, 1 in Nature Commun. and 17 invited reviews, cited > 12 600 times in the literature (h-index = 57). He has presented > 45 plenary and invited lectures at international conferences and > 90 invited seminars in universities and research institutions around the world. He has led more than 50 projects with international companies, incl. Unilever, BASF, PepsiCo, Saint Gobain, Wacker, Dow Corning and Heineken, and is a co-inventor of 14 filed and granted patents. Prof. Denkov has been a supervisor and co-supervisor of 12 completed PhD Theses, and 2 other Theses are under preparation. He is a member of the Physical Sciences working group in European Space Agency (ESA) and of the Council of the International Association of Colloid and Interface Scientists (IACIS). He is a member of the Physical Sciences working group in European Space Agency (ESA) and of the Council of the International Association of Colloid and Interface Scientists (IACIS).
For his research achievements, in 2019 Prof. Denkov was awarded the Solvay Prize of the European Colloid and Interface Society (ECIS) and was elected as a regular member of Academia Europaea. For his outstanding research contributions, he has received numerous awards, including the “Pythagoras” Award, the highest Bulgarian recognition for scientific achievements (2010), the Solvay Prize of the European Colloid and Interface Society (2019), the Lectureship Award from the Division of Colloid and Surface Chemistry in Japan (2020), and the Prix Formula – Pierre Fillet Prize of the French Chemical Society (2025). Based on their publications and citations received in the past year only, Prof. Denkov has been ranked 395 in Chemical Physics category among 115 551 scientists included in the Stanford/Elsevier’s Top 2% Scientists Ranking in 2025.
Publications
Most recent publications
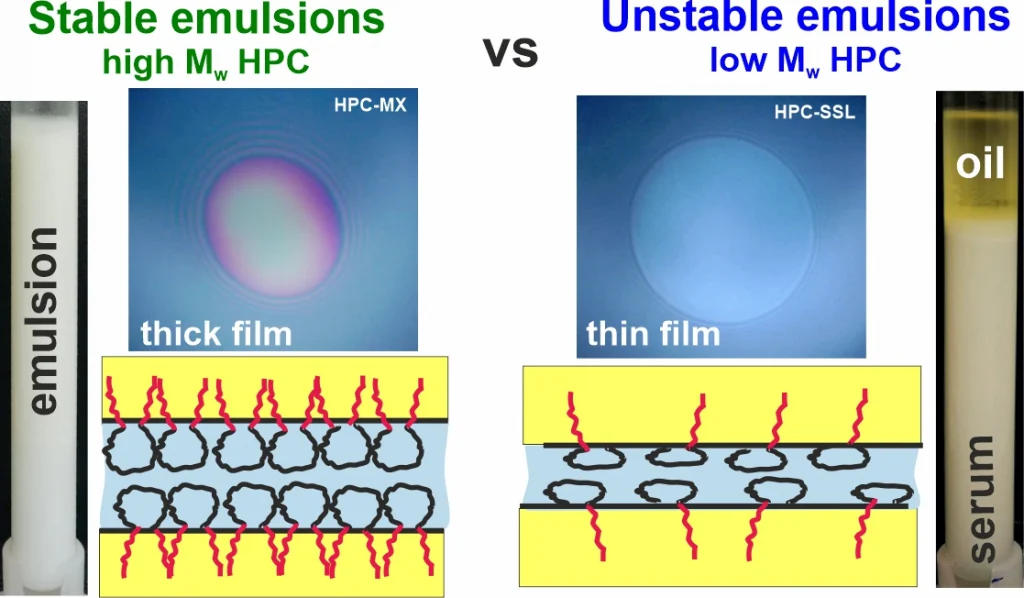
Hydroxypropyl cellulose polymers as efficient emulsion stabilizers: The effect of molecular weight and overlap concentration
Hydroxypropyl cellulose (HPC) is a non-digestible water-soluble polysaccharide used in various food, cosmetic, and pharmaceutical applications. In the current study, the aqueous solutions of six HPC grades, with molecular mass ranging from 40 to 870 kDa, were characterized with respect to their precipitation temperatures, interfacial tensions (IFTs), rheological properties and emulsifying and stabilization ability in palm (PO) and sunflower (SFO) oil emulsions. The main conclusions from the obtained results are as follows: (1) Emulsion drop size follows a master curve as a function of HPC concentration for all studied polymers, indicating that polymer molecular mass and solution viscosity have a secondary effect, while the primary effect is the fraction of surface-active molecules, estimated to be around 1–2% for all polymers. (2) Stable emulsions were obtained only with HPC polymers with Mw ≥ 400 kDa at concentrations approximately 3.5 times higher than the critical overlap concentration, c*. At PO concentrations beyond 40 wt. % or when the temperature was 25 °C, these emulsions appeared as highly viscous liquids or non-flowing gels. (3) HPC polymers with Mw < 90 kDa were unable to form stable emulsions, as the surface-active molecules cannot provide steric stabilization even at c ≳ 4–5 c*, resulting in drop creaming and coalescence during storage.
Rheology of dispersions containing non-spherical lipid particles
The rheological properties of disperse systems play a crucial role in the production of foods, cosmetics, and pharmaceuticals with desired characteristics. Emulsion viscosity can be increased through various methods, incl. increasing the oil volume fraction, incorporating rheological modifiers, or inducing partial coalescence between the droplets. It is well known that suspensions containing inorganic non-spherical particles often exhibit significantly higher viscosities when compared to those with spherical particles. The spontaneous drop self-shaping phenomenon in emulsions, first reported in detail by Denkov et al. (Nature, 2015, 528, 392–395), enables the formation of fluid and frozen lipid particles with regular non-spherical shapes, including platelets, rods and fibers. In this study, we utilize this approach to prepare emulsions containing non-spherical frozen particles of various shapes and investigate their rheological properties. The effects of oil volume fraction, surfactant type, initial drop size and polydispersity are investigated. The results reveal that non-flowing, gel-like samples can be prepared at ca. 11 vol% oil fraction when the emulsion contains polydisperse droplets which acquire non-spherical shapes upon cooling. For comparison, more than ca. 65 vol% oil is needed to obtain similar rheological characteristics in samples containing spherical particles. Additionally, we demonstrate that the optimal drop size for gel preparation is d32 ≈ 4–13 μm. The obtained results are explained mechanistically, and guiding principles are provided for preparing emulsions with increased viscosities using this new approach.

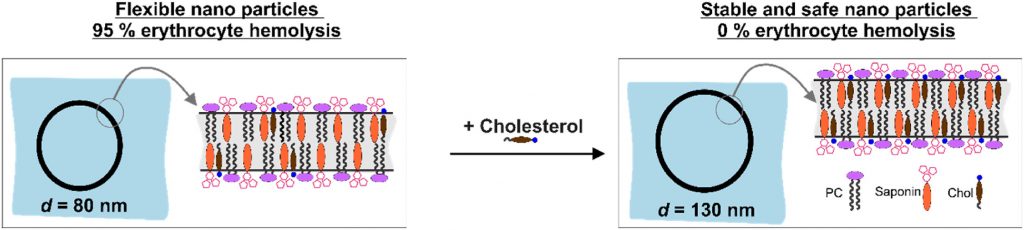
ISCOM-type matrix from beta-escin and glycyrrhizin saponins
Background and aims
Nanotechnology provides the opportunity for construction of modern transport devices such as nanoparticles for a variety of applications in the field of medicine. A novel experimental protocol for the formation of saponin-cholesterol-phospholipid nanoparticles of vesicular structure has been developed and applied to prepare stable nanoparticles using escin or glycyrrhizin as saponins.
Methods
The methods for nanoparticle construction include a sonication at 90 °C of the initial mixture of components, followed by an additional sonication on the next day for incorporation of an additional amount of cholesterol, thus forming stable unilamellar vesicles. Tests and assays for cell viability, erythrocyte hemolysis, flow cytometry, and fluorescent microscopy analyses have been performed.
Results
By selecting appropriate component ratios, stable and safe particles were formulated with respect to the tested bio-cells. The prepared nanoparticles have mean diameter between 70 and 130 nm, depending on their composition. The versatility of these nanoparticles allows for the encapsulation of various molecules, either within the vesicle interior for water-soluble components or within the vesicle walls for hydrophobic components. The saponin particles formed after cholesterol post-addition (E3-M2) are stable and 100 % of the cells remain viable even after 10-times dilution of the initial particle suspension. These particles are successful included into isolated mouse macrophages.
Conclusions
Among the variety of generated nanoparticles, the E3-M2 particles demonstrated properties of safe and efficient devices for future vaccine design and antigen targeting to immune system.
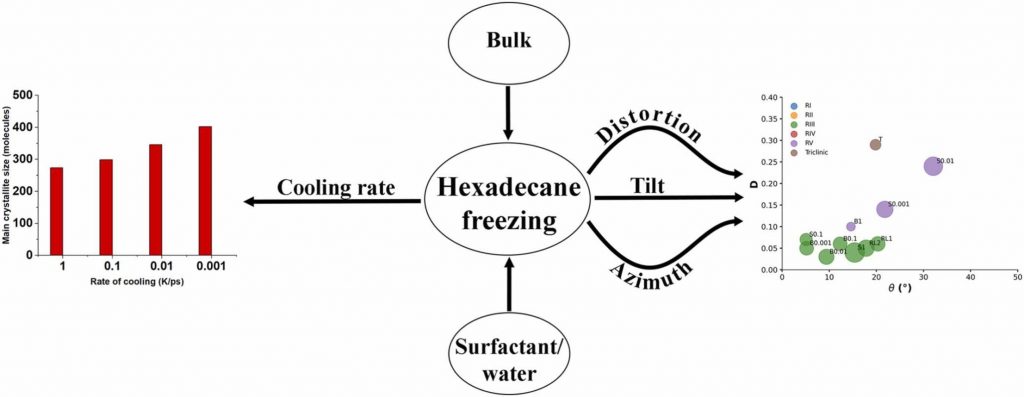
Polymorphism in triglyceride systems: in bulk and in emulsion droplets
Types of phases obtained by molecular dynamics simulations upon freezing of hexadecane-containing systems
Medium- to long-chain alkanes can form upon cooling intermediate phases between isotropic liquid and solid crystalline, called rotator phases, where relative freedom of the molecules to rotate about their long axis is combined with long range translational order. Rotator phases are well documented experimentally but the mechanism of their formation at the molecular level is still not fully explained. In a previous work, we have shown that molecular dynamics simulations can produce rotator phases upon cooling of hexadecane [S. Iliev et al., J. Col. Int. Sci., 2023, 638, 743]. The aim of the current work is to develop a procedure to identify the specific ordered phase obtained in the simulations. The influence of the cooling rate on the freezing process of hexadecane (bulk and surfactant-interfaced to water) is tested as well. Several parameters are combined to quantify the degree of ordering and the type of phase in the studied systems. These are the tilt angle of the molecules with respect to the crystallite plane, the radial distribution function of the centre of mass of the molecules in the crystallite, the percentage of the gauche torsion angles in the molecules, the angle of the second principal axis of each molecule with respect to the x axis of the coordinate system, and estimates from Voronoi analysis. The results show that the systems form a rotator phase, which transitions gradually towards the thermodynamically most stable triclinic crystal, and the transformation progresses to different extent depending on the system. The influence of the cooling rate is related only to the size of the largest crystallite formed, the other parameters of the freezing process remain unaffected. The work also presents a robust procedure for obtaining and identifying different types of ordered phases in alkane-containing systems with thoroughly tested computational protocol and a comprehensive set of structural analyses. Several key characteristics are advanced, compared to previous research [Ryckaert et al., Mol. Phys., 1989, 67, 957; Wentzel et al., J. Chem. Phys. 2011, 134, 224504], namely, a new methodology is proposed to compute the unit cell deformation parameter and azimuthal angle from MD simulation trajectories of the freezing process in alkane-containing systems. The suggested structural analysis, which is independent of the coordinate system, is applicable to any linear-chain system with polycrystalline structure.