Nikola Genchev, MSc Student
Interests
- Foams and antifoams
- Molecular dynamics simulations
Publications
Most recent publications
Understanding drug solubilization in intestinal mixed micelles through molecular dynamics simulations
Hypothesis
Solubilization is a fundamental process that underpins various technologies in the pharmaceutical and chemical industry. However, knowledge of the location, orientation and interactions of solubilized molecules in the micelles is still limited. We expect all-atom molecular dynamics simulations to improve the molecular-level understanding of solubilization and to enable its in silico prediction.
Methods
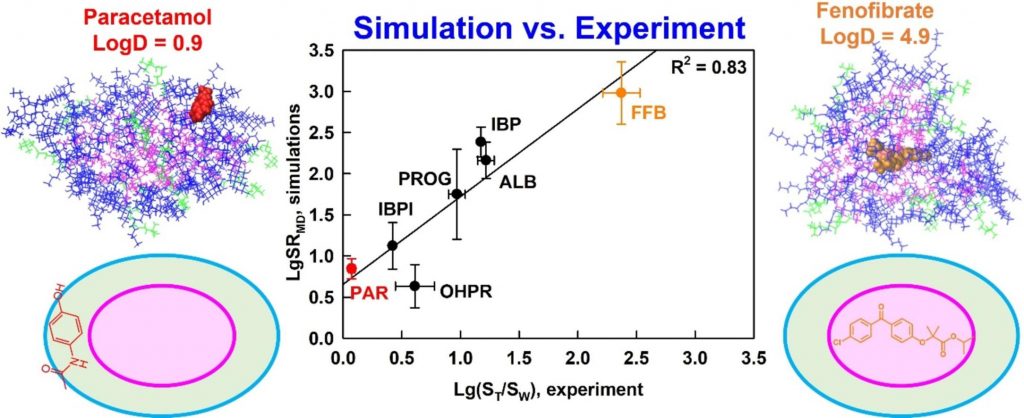
The solubilization of six drugs in intestinal mixed micelles composed of taurocholate and dioleoyl phosphatidylcholine was simulated by molecular dynamics in explicit water and measured experimentally by liquid chromatography. The location and orientation of the solubilized drugs were visualized by cumulative radial distribution functions and interactions were characterized by radial distribution function ratios and hydrogen bonding.
Findings
A new simulation-derived parameter was defined, which accounts for drug-micelle and drug-water interactions and correlates (R2 = 0.83) with the experimentally measured solubilization. Lipophilicity was found to govern the location of all drugs in the micelle (hydrophobic core, palisade layer or on the surface), while hydrogen bonding was crucial for orientation and solubilization of two of the molecules. The study demonstrates that explicit, hydrogen bond-forming water molecules are vital for accurate prediction of solubilization and provides a comprehensive framework for quantitative studies of drug location and orientation within the micelles.
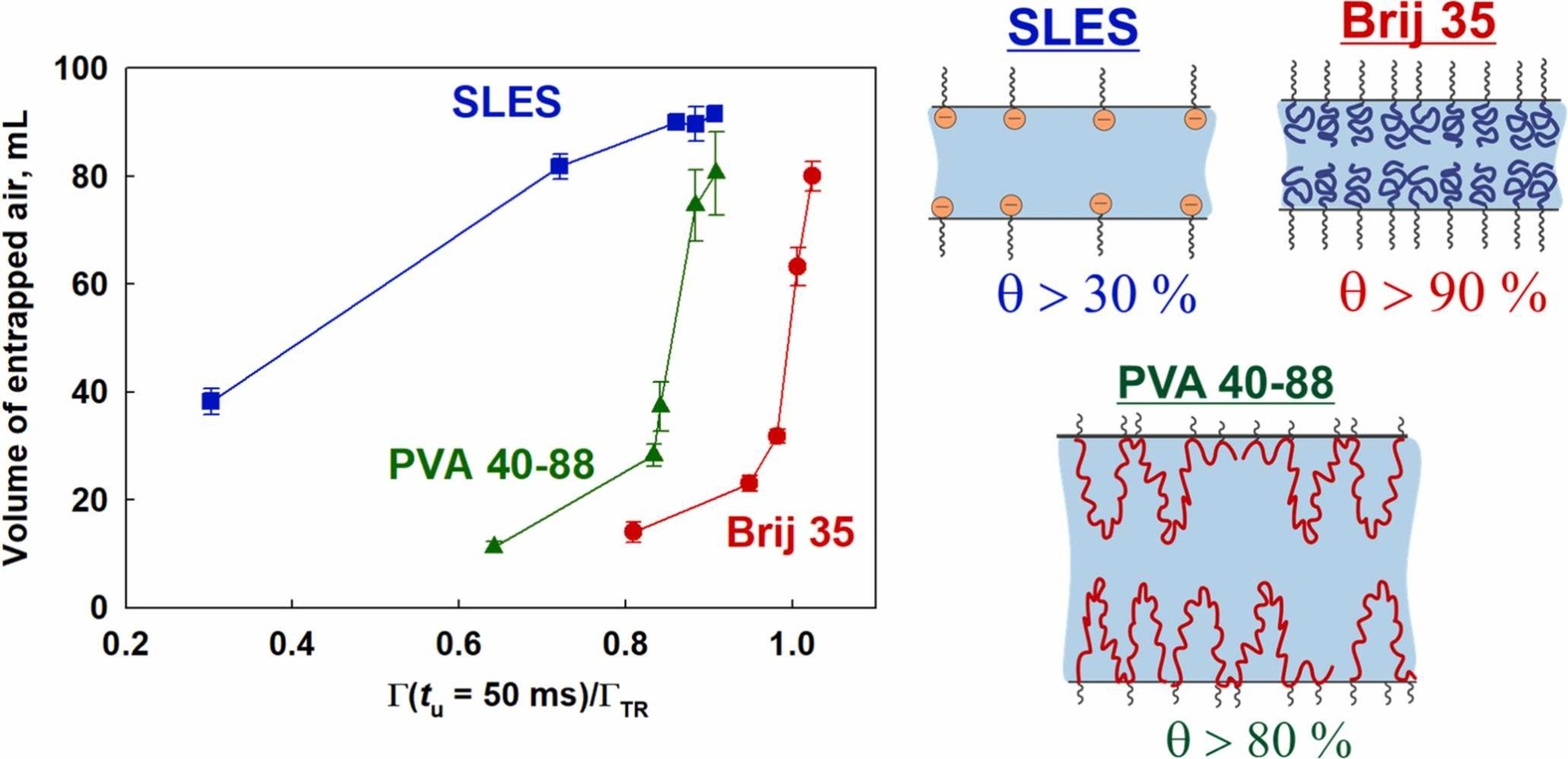
Surface and foam properties of polyvinyl alcohol solutions
The surface, film, and foam properties of six polyvinyl alcohols (PVA) with different degrees of hydrolysis (DH) and molecular weights were studied and compared with the properties of nonionic Brij 35 and anionic SLES. Four different foaming methods were employed: the fast foaming method (Bartsch test), intermediate tests (shake test and Ultra Turrax), and slow foaming method (foam rise method) to assess the foamability at various bulk concentrations. The foamability data obtained from different foaming tests, utilizing various surfactant and polymeric concentrations, and differing foaming times, were shown to follow a universal master curve when plotted as relative foamability vs. scaled concentration. A new simple theoretical equation was derived to describe this universal curve, allowing for foamability prediction. The threshold surfactant concentration required to achieve 50% of the maximal foam volume under given conditions (used for scaling the bulk concentration) was found to decrease with foaming time and increase from slow foaming methods to fast foaming methods. When the experimental data are plotted against surface coverage, the results for PVA solutions exhibit intermediate behavior between nonionic surfactants, where a threshold surface coverage of 95% is required to achieve 50% of maximal foamability and anionic surfactants, where 30% surface coverage is sufficient to reach 50% of maximal foamability due to the action of electrostatic repulsion. This intermediate behavior observed in PVA solutions is attributed to the presence of a long-range steric repulsion arising from the adsorption of PVA molecules onto the bubble surfaces. This work advances the foam field by showing that the approach developed in Petkova et al. 2020 can be used for polymeric molecules and by deriving a new equation for foamability which is expected to be applicable for wide range of systems.