Nevena B. Pagureva, Ph.D.
Interests
- Interfacial Properties of Natural Surfactants
- Surface and Bulk Rheology of Monolayers and Foams
- Foam Films
Publications
Most recent publications
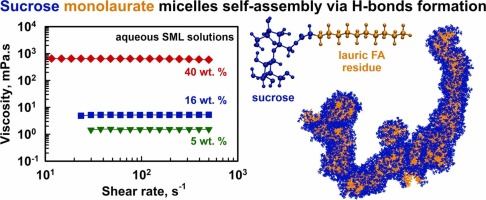
Sucrose monolaurate self-assembly via hydrogen bonding: role of surfactant concentration and urea
Sugar esters, a class of surfactants derived from renewable resources, have attracted significant attention due to their biodegradability, low toxicity, and broad applications in food, cosmetic, and pharmaceutical formulations. Despite their widespread use, the phase behavior of these compounds in aqueous systems remains incompletely understood. In this study, we investigate the self-assembly of a nonionic sucrose ester of lauric acid in 1–40 wt% concentration range using rheological measurements, dynamic light scattering, X-ray scattering, DOSY NMR, and molecular dynamics simulations. Formation of spherical micelles with a diameter of 5.4 nm is observed at low surfactant concentrations, driven by hydrophobic interactions between the alkyl tails. These solutions exhibit Newtonian flow behavior with viscosities close to that of pure water. However, the viscosity increases from 5 mPa.s at 16 wt% to 640 mPa.s at 40 wt%, while the Newtonian character persists even at 40 wt%. This behavior is explained with the formation of interconnected, thread-like micellar structures of (almost) spherical micelles that largely preserve their distinctiveness, resembling the “pearl necklace” arrangement known for polymer systems. The main driving force for this supramolecular organization was found to be the hydrogen bonding between sucrose headgroups. The addition of 6 M urea, a known hydrogen bond disruptor, significantly reduces micelle clustering and the viscosity decreases to 150 mPa.s at 40 wt% concentration, supporting the proposed aggregation mechanism. These findings contribute to a deeper understanding of the self-assembly behavior of sucrose esters in aqueous environment and highlight their potential for controlled aggregation in practical formulations.
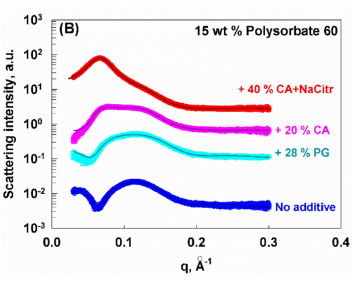
Role of food additives on properties of Polysorbate 60 solutions
Polysorbates are hydrophilic, nonionic surfactants widely used in food, cosmetic, and pharmaceutical products. Often, these emulsifiers are used in combination with different preservatives, and it is essential to conduct an in-depth study of the influence of such additives on the properties of Polysorbate 60 solutions. In the current study, we investigated the effect of various food additives: citric acid, sodium benzoate, potassium sorbate, a mixture of citric acid and sodium citrate, and propylene glycol on the stability and micellar properties of Polysorbate 60 by using different experimental methods such as GC, DSC, SAXS, DLS, optical observations in polarised light, and NMR. When stored at room temperature without additives, solutions of Polysorbate 60 slowly undergo phase separation over time. Our results show that citric acid, a mixture of citric acid and sodium citrate, and propylene glycol increase the rate of this phase separation. In contrast, sodium benzoate and potassium sorbate are incorporated into the mixed micelles. They localize within the palisade layer, a region of the micelle where the surfactant tails begin, effectively preventing the phase separation. As a result, Polysorbate 60 solutions with these specific additives remain transparent and stable for over a year
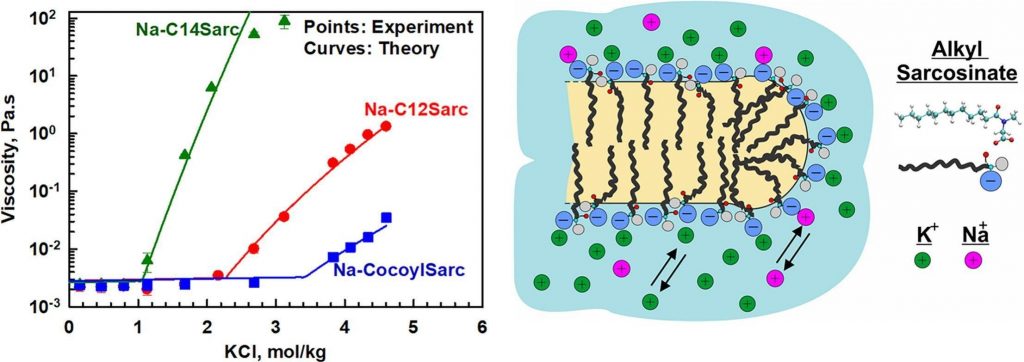
Role of electrolytes and co-surfactants on the rheological properties of sodium N-acyl sarcosinate solutions
Alkyl sarcosinates are amino acid-based anionic surfactants commonly used as primary surfactants in sulfate-free personal care products. The major aim of this study is to identify the key factors influencing the rheological behaviour of sodium sarcosinate solutions and their mixtures with nonionic, zwitterionic, and cationic co-surfactants. To achieve this, we examined the effects of salt type and concentration for alkyl sarosinates with different chain lengths (dodecyl, tetradecyl, and cocoyl) across concentration range of 2–20 wt%. Experimental results reveal two distinct regions in the salt curve for the three studied sarcosinates. At low electrolyte concentrations, viscosity remains constant until reaching the critical electrolyte concentration, C1, beyond which viscosity increases logarithmically with salt concentration. Further electrolyte addition leads to phase separated solutions at the critical precipitation concentration, CTR. Both C1 and CTR decrease as the hydrocarbon chain length increases from dodecyl to tetradecyl. However, the presence of shorter chain molecules in cocoyl sarocisinate significantly increases both C1 and CTR due to the formation of spherical micelles. A theoretical expression for predicting viscosity dependence on salt concentration is derived and successfully applied to describe the experimental data. The adsorption energy of sodium and potassium to alkyl sacrosinate micelle surfaces is found to be much smaller than that to sodium lauroyl ether sulfate surfactants (1 vs. 3 kBT for Na+ and 0.8 vs. 3.8 kBT for K+). No significant effect of amphoteric co-surfactants, including cocoamidopropyl betaine, sulfobetaine, or decylamine oxide, is observed. NMR analysis confirms that cocoamidopropyl betaine and sodium dodecyl sarcosinate form mixed micelles that are structurally similar to sarcosinate micelles, as carboxyl groups remain exposed on the micelle surfaces in both cases. When using amine oxide and sulfobetaine, the increase in viscosity is attributed to the elongation of mixed micelles, though steric hindrance from side methyl groups limits their growth. The practical significance of this study lies in the finding that longer-chain alkyl sarcosinates (such as tetradecyl, as investigated here) can attain significantly higher viscosities at lower salt concentrations compared to shorter-chain analogs or surfactant mixtures. The scientific significance stems from the development of a theoretical model capable of predicting the viscosity of alkyl sarcosinate solutions across various surfactant and salt concentrations.
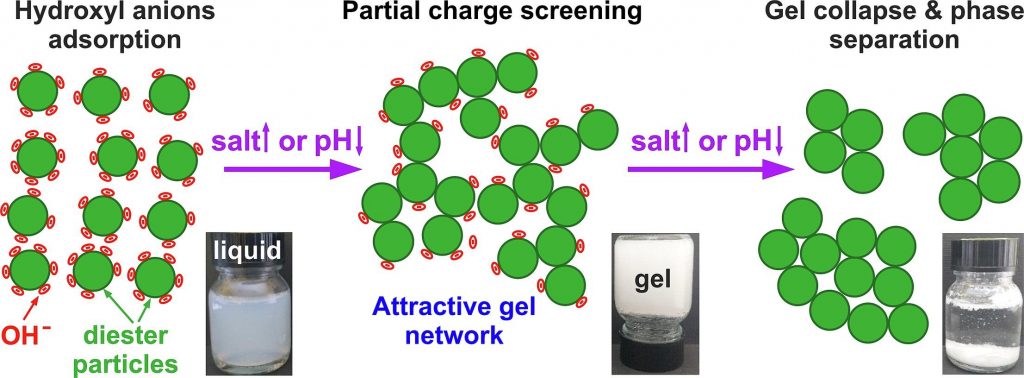
Salt-induced gelation of nonionic sucrose ester dispersions
Hypothesis
The dispersions of nonionic sucrose ester surfactants in water exhibit a highly negative zeta-potential, though its origin remains controversial. The addition of electrolytes to these dispersions may influence their zeta-potential, thus potentially affecting their physicochemical properties.
Experiments
The electrolyte- and pH- driven gelation of aqueous dispersions of commercial sucrose stearate (S970) containing ca. 1:1 monoesters and diesters was studied using optical microscopy, rheological and zeta-potential measurements, and small-angle X-ray scattering techniques.
Findings
At low electrolyte concentrations and pH ≳ 5, 0.5–5 wt% S970 dispersions exhibited low viscosities and behaved as freely flowing liquids. The addition of electrolytes of low concentrations, e.g. 9 mM NaCl or 1.5 mM MgCl2, induced the formation of a non-flowing gels. This sol–gel transition occurred due to the partial screening of the diesters particles charge, allowing the formation of an attractive gel network, spanning across the dispersion volume. Complete charge screening, however, led to a gel-sol transition and phase separation. Gel formation was observed also by pH variation without electrolyte addition, whereas the addition of free fatty acids had negligible impact on dispersion properties. These findings support the hypothesis that the negative charge in sucrose ester dispersions arises from hydroxyl anions adsorption on particles surfaces. Gels were formed using just 1.3 wt% surfactant, and the critical electrolyte concentration for gelation was found to scale approximately with the square of the cation charge, in agreement with the low surface charge density theory. The biodegradable sucrose esters gels offer a sustainable alternative for structuring personal and home care products, replacing the wormlike micelles of synthetic surfactants typically used at much higher surfactant and salt concentrations.
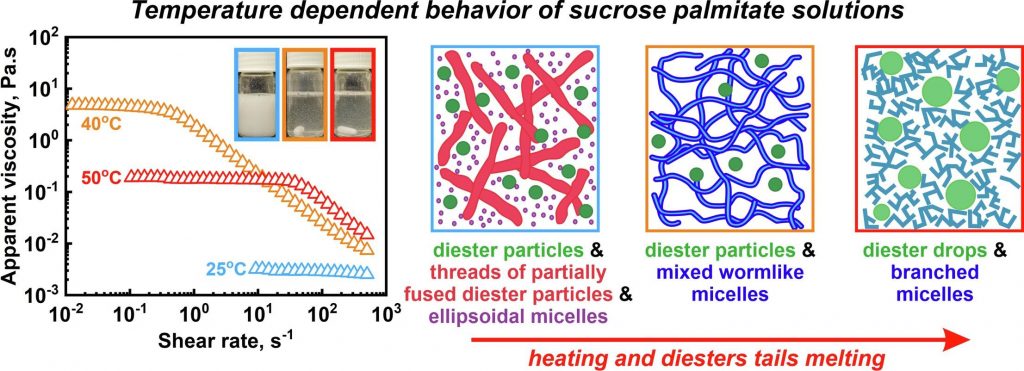
Temperature response of sucrose palmitate solutions: Role of ratio between monoesters and diesters
Hypothesis: Aqueous solutions of long-chain water-soluble sucrose ester surfactants exhibit non-trivial response to temperature variations, revealing a peak in viscosity around 40–50 ◦C. While previous investigations have explored the structures within sucrose stearate systems at various constant temperatures, a comprehensive understanding of the entire temperature dependence and the underlying molecular factors, contributing to this phenomenon is currently missing. Experiments: Temperature dependent properties and supramolecular structures formed in aqueous solutions of commercial sucrose palmitate were examined using SAXS/WAXS, DSC, optical microscopy, rheological measurements, NMR, and cryo-TEM. Findings: The underlying mechanism governing this unusual behavior is revealed and is shown to relate to the mono- to di-esters ratio in the solutions. Solutions primarily containing sucrose monoesters (monoesters molecules ≳ 98% of all surfactant molecules) exhibit behavior typical of nonionic surfactants, with minimal changes with temperature. In contrast, the coexistence of mono- and di-esters results in the formation of discrete monodisperse diester particles and a network of partially fused diester particles at low temperature. As the temperature approaches the diesters’ melting point, wormlike mixed micelles form, causing a viscosity peak. The height of this peak increases significantly with the diester concentration. Further temperature increase leads to fluidization of surfactant tails and formation of branched micelles, while excess diester molecules phase separate into distinct droplets.