Nadya I. Politova, Ph.D.
Interests
- Foaming and foam rheology
- Physicochemical control of foam properties
- Emulsification and emulsion stability
Publications
Most recent publications
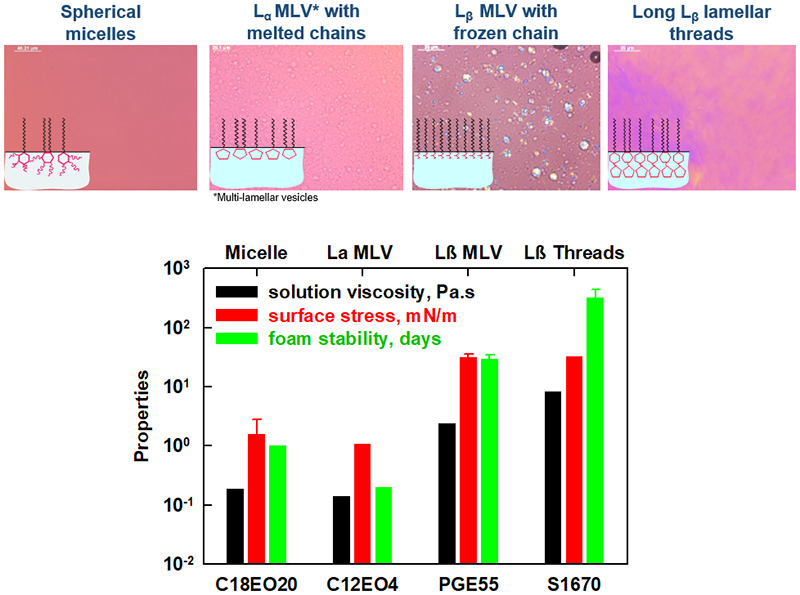
Interplay between bulk aggregates, surface properties and foam stability of nonionic surfactants
In our previous study (Mustan et al. 2021) we showed that foams formed from two oil-soluble nonionic surfactants (Span 60 and Brij 72) can remain stable for more than 10 days at room temperature at high sugar concentration. The major aim of the current study is to reveal the interrelation between the surfactant structure and foam stability by investigating 6 polyoxyethelene alkyl ethers and 12 fatty acid esters with a wide variety of hydrophobic chain lengths (C12; C16; C18 and C18:1) and hydrophilic head-groups (sorbitol, glycerol, sucrose). Foams stable for more than 100 days at room temperature are obtained when sucrose palmitate or stearate (P1670 or S1670) are used as surfactants. This exceptional foam stability is related to the gelation of the aqueous phase and to the formation of solid adsorption layer with zero surface tension upon compression, thus preventing water drainage and decelerating the bubble Ostwald ripening. The foam stability decreases with (i) increasing the number of EO groups in polyoxyethylene alkyl ethers and in fatty acid sorbitan esters; (ii) decreasing the number of C-atoms in the surfactant tail for all studied surfactants; (iii) addition of double bond in the surfactant tail. The lower foam stability in all three cases is related to the worse packing of the surfactant molecules within the adsorption layer, leading to faster Ostwald ripening and subsequent bubble coalescence. The diesters present as admixture in the fatty acid esters play an important role in the foam stabilization by further compacting the adsorption layers and lowering the rate of Ostwald ripening. These conclusions can be used as a predictive tool for surfactant selection in the development of food or pharmaceutical foam concentrates that can be diluted before final use.
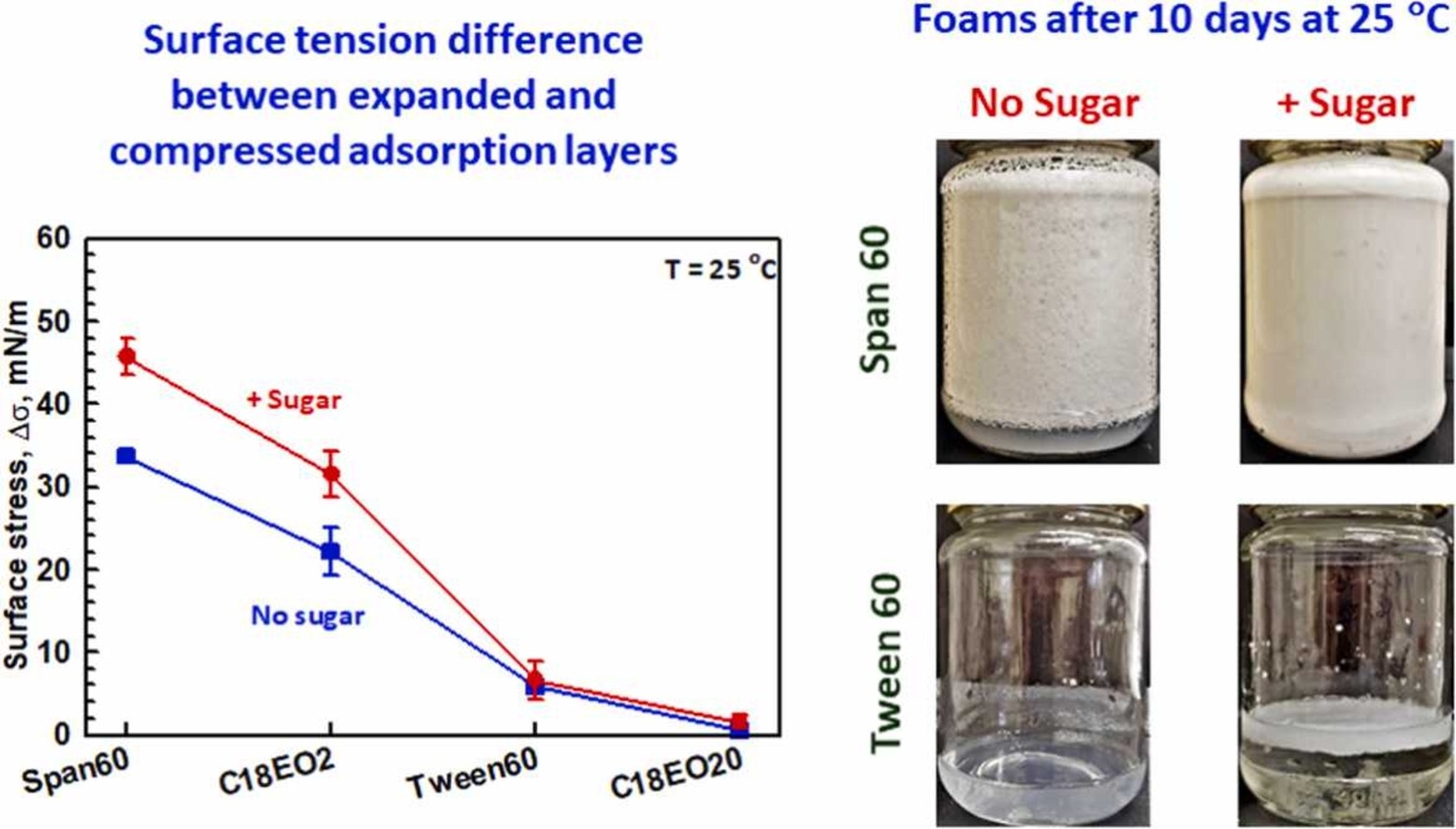
Oil soluble surfactants as efficient foam stabilizers
The surface and foam properties of two oil-soluble surfactants, Span 60 and Brij 72, and two water-soluble surfactants, Tween 60 and Brij S20, are compared. Aqueous surfactant solutions containing sugar at high concentration (63 wt%) are also studied in the context of sweet food-related foams. The experimental results show that foams with very small bubbles of ca. 5 µm are formed and remain stable for more than 10 days at room temperature when oil-soluble surfactants are dispersed in the sugar-rich solutions. The excellent stability of these foams is related to (1) The multilamellar vesicles present in the respective surfactant dispersions which increase the viscosity and decrease the rate of water drainage from the foam; (2) The very slow exchange of surfactant molecules between the interface and the adjacent aqueous solution that leads to very significant difference in the surface tension of shrinking and expanding bubbles, which in turn decreases the rate of bubble Ostwald ripening. The foams formed from Span 60 are more stable as compared to the foams formed from Brij 72, due to the higher viscosity and the slower interface-solution exchange. The foams formed from Brij S20 solutions are least stable – they are completely destroyed after 1 day at room temperature, even in the presence of sugar. The main conclusion of this study is that oil soluble surfactants can form condense adsorption layers on the bubble surfaces after adsorption from aqueous solutions with and without sugar in it. The formed layers, which present a better molecular packing, reduce the gas permeability and decelerate the Ostwald ripening disproportionation of the foam This is obtained by a relatively low interfacial tension imparted by continuous shrinkage and expansion of bubbles during foam generation, ultimately ensuring long-term stabilization of formed foams.
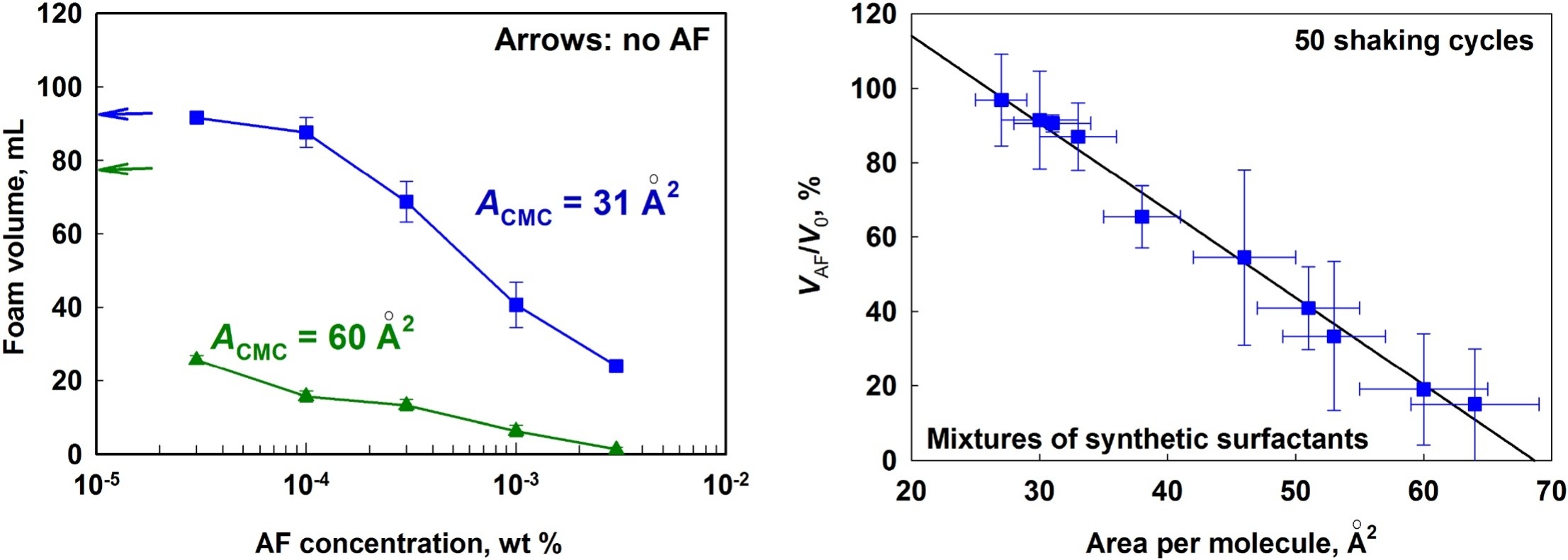
Role of surfactant adsorption and surface properties for the efficiency of PDMS-silica antifoams
We study how the composition of various surfactant mixtures affects the efficiency of mixed PDMS–silica antifoam in foamed surfactant solutions. First, systematic experiments are performed to characterize the surface and foam film properties of the studied surfactant solutions. The spreading, bridging and entry coefficients are calculated and the spreading ability of the antifoam is characterized by microscopy observations and by surface tension measurements. Next, the initial antifoam activity and the antifoam durability are characterized in foam tests. The obtained results reveal that the antifoam efficiency in solutions of low-molecular mass surfactants with low surface dilatational modulus depends strongly on the density (area-per-molecule) of the respective adsorption layer. The addition of nonionic surfactants, which increase the mean area-per-molecule in the mixed adsorption layer, enhances significantly the antifoam activity and durability. In contrast, the addition of surfactants, which decrease the mean area-per-molecule, suppresses the antifoam activity. Furthermore, we found that surfactant mixtures which form condensed adsorption layers on the solution surface suppress strongly the antifoam activity. As an extreme, the condensed adsorption layer formed from the natural surfactant Quillaja saponin suppresses the antifoam spreading even at highly positive spreading coefficient which results also in very poor AF efficiency. The obtained results rationalize in a coherent way the observed differences in the AF activity and durability in mixed solutions of various ionic, nonionic and zwitterionic surfactants.
Physicochemical control of foam properties
This article summarizes our recent understanding on how various essential foam properties could be controlled (viz. modified in a desired way) using appropriate surfactants, polymers, particles and their mixtures as foaming agents. In particular, we consider the effects of these agents on the foaminess of solutions and suspensions (foam volume and bubble size after foaming); foam stability to liquid drainage, bubble coalescence and bubble Ostwald ripening; foam rheological properties and bubble size in sheared foams. We discuss multiple, often non-trivial links between these foam properties and, on this basis, we summarize the mechanisms that allow one to use appropriate foaming agents for controlling these properties. The specific roles of the surface adsorption layers and of the bulk properties of the foaming solutions are clearly separated. Multiple examples are given, and some open questions are discussed. Where appropriate, similarities with the emulsions are noticed.
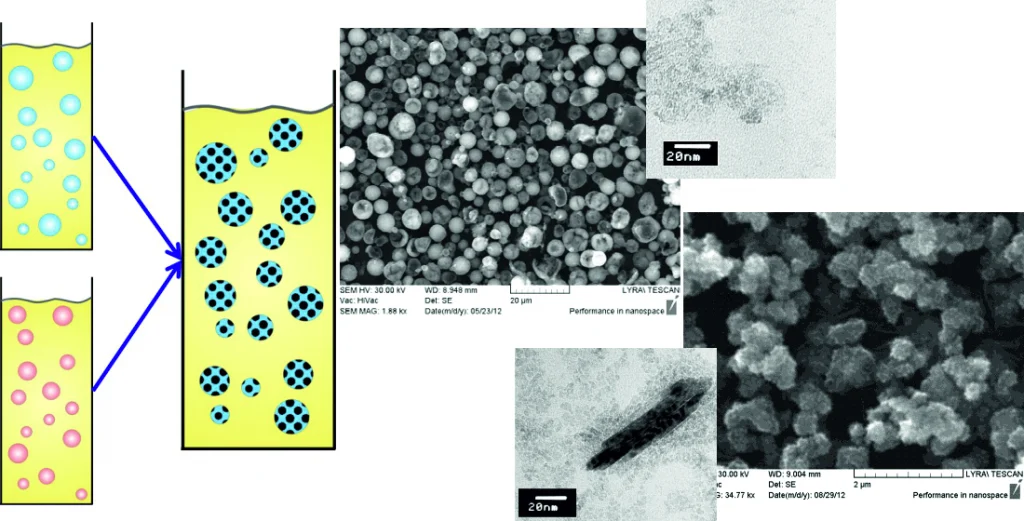
Preparation of TiO2 nanoparticle aggregates and capsules by the ‘two-emulsion method’
TiO2-based materials are of great practical interest in several technological areas. Both the size and the morphology of the TiO2 particles are of critical importance for their applications. The current study explores the effect of several factors on the outcome of the TiO2 particle synthesis via the so-called ‘two-emulsion method’. In this technique, two water-in-oil emulsions—each of them containing different reactant in the dispersed water drops—are mixed under well controlled conditions. Upon such mixing, partial coalescence of the water drops from the two emulsions leads to mixing of the drop content, with chemical reaction occurring within the drops, and to synthesis of Ti(OH)4 particles. Afterwards, the latter are transformed by emulsion heating into TiO2 particles and aggregates of predominantly anatase structure. Our results show that—depending on the precursor and surfactant concentrations, oil viscosity, emulsification time, and mixing speed—the obtained nanoparticles could aggregate either on the drop surface, forming capsules with a very smooth surface, or inside the water droplets, thus leading to hierarchically structured aggregates of micrometer size. The spherical smooth capsules are constructed of very small monodisperse TiO2 nanoparticles with size below 5 nm. The hierarchical bulk aggregates, on the other hand, are formed from bigger primary particles of sub-micrometer size. The obtained results show that one can obtain various TiO2 structures by controlling the conditions during the emulsion preparation and mixing.