Monika I. Hristova, Ph.D. Candidate
Interests
- Surfactant- and Particle-Stabilized Foams
- Bulk Rheology of Suspensions and Foams
- Macroporous Ceramics
Publications
Most recent publications
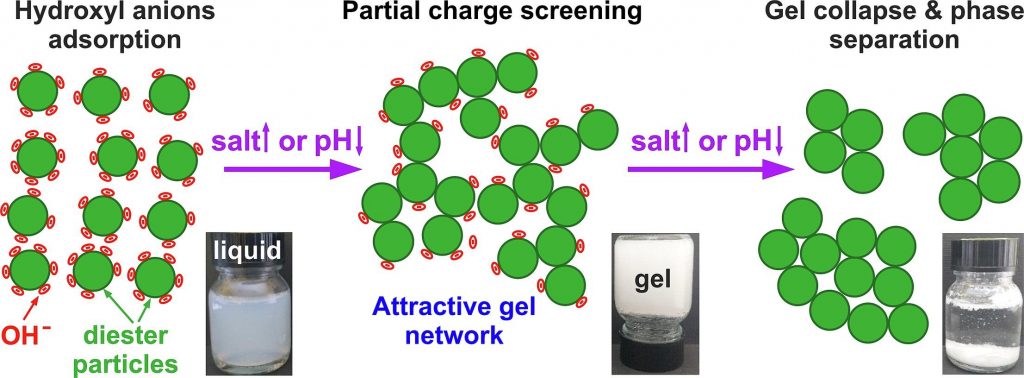
Salt-induced gelation of nonionic sucrose ester dispersions
Hypothesis
The dispersions of nonionic sucrose ester surfactants in water exhibit a highly negative zeta-potential, though its origin remains controversial. The addition of electrolytes to these dispersions may influence their zeta-potential, thus potentially affecting their physicochemical properties.
Experiments
The electrolyte- and pH- driven gelation of aqueous dispersions of commercial sucrose stearate (S970) containing ca. 1:1 monoesters and diesters was studied using optical microscopy, rheological and zeta-potential measurements, and small-angle X-ray scattering techniques.
Findings
At low electrolyte concentrations and pH ≳ 5, 0.5–5 wt% S970 dispersions exhibited low viscosities and behaved as freely flowing liquids. The addition of electrolytes of low concentrations, e.g. 9 mM NaCl or 1.5 mM MgCl2, induced the formation of a non-flowing gels. This sol–gel transition occurred due to the partial screening of the diesters particles charge, allowing the formation of an attractive gel network, spanning across the dispersion volume. Complete charge screening, however, led to a gel-sol transition and phase separation. Gel formation was observed also by pH variation without electrolyte addition, whereas the addition of free fatty acids had negligible impact on dispersion properties. These findings support the hypothesis that the negative charge in sucrose ester dispersions arises from hydroxyl anions adsorption on particles surfaces. Gels were formed using just 1.3 wt% surfactant, and the critical electrolyte concentration for gelation was found to scale approximately with the square of the cation charge, in agreement with the low surface charge density theory. The biodegradable sucrose esters gels offer a sustainable alternative for structuring personal and home care products, replacing the wormlike micelles of synthetic surfactants typically used at much higher surfactant and salt concentrations.
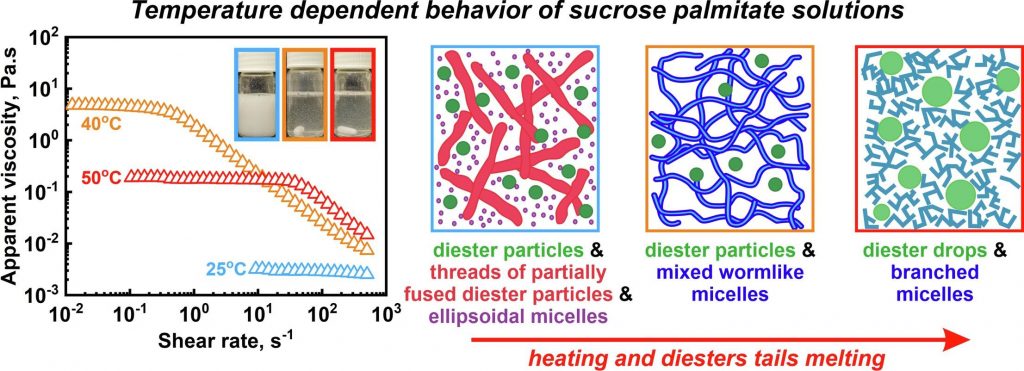
Temperature response of sucrose palmitate solutions: Role of ratio between monoesters and diesters
Hypothesis: Aqueous solutions of long-chain water-soluble sucrose ester surfactants exhibit non-trivial response to temperature variations, revealing a peak in viscosity around 40–50 ◦C. While previous investigations have explored the structures within sucrose stearate systems at various constant temperatures, a comprehensive understanding of the entire temperature dependence and the underlying molecular factors, contributing to this phenomenon is currently missing. Experiments: Temperature dependent properties and supramolecular structures formed in aqueous solutions of commercial sucrose palmitate were examined using SAXS/WAXS, DSC, optical microscopy, rheological measurements, NMR, and cryo-TEM. Findings: The underlying mechanism governing this unusual behavior is revealed and is shown to relate to the mono- to di-esters ratio in the solutions. Solutions primarily containing sucrose monoesters (monoesters molecules ≳ 98% of all surfactant molecules) exhibit behavior typical of nonionic surfactants, with minimal changes with temperature. In contrast, the coexistence of mono- and di-esters results in the formation of discrete monodisperse diester particles and a network of partially fused diester particles at low temperature. As the temperature approaches the diesters’ melting point, wormlike mixed micelles form, causing a viscosity peak. The height of this peak increases significantly with the diester concentration. Further temperature increase leads to fluidization of surfactant tails and formation of branched micelles, while excess diester molecules phase separate into distinct droplets.
Role of particle size on the cohesive strength of non-sintered (green) ceramics
Preparation of particle-loaded foams, followed by drying, sintering and/or cross-linking are widely explored routes for developing lightweight ceramics with high mechanical strength. The non-sintered dry ceramic foams are less studied due to their intricate production and the assumed poor mechanical strength of the obtained “green” materials. Here we produce lightweight ceramics from foamed particle suspensions containing spherical silica particles with radii varied between 4.5 nm and 7 µm. The wet foams are prepared in the presence of cationic surfactant and were dried at ambient conditions to obtain porous materials with mass densities between 100 and 700 kg/m3. The materials containing smaller particles exhibited much higher strength (by up to 2000 times), approaching that of the sintered materials. A new theoretical expression for predicting the mechanical strength of such materials is derived and is used to explain the measured strengths of the produced materials through the van der Waals attraction between the particles in the final dry materials.
 ceramics-1024x410.jpg)
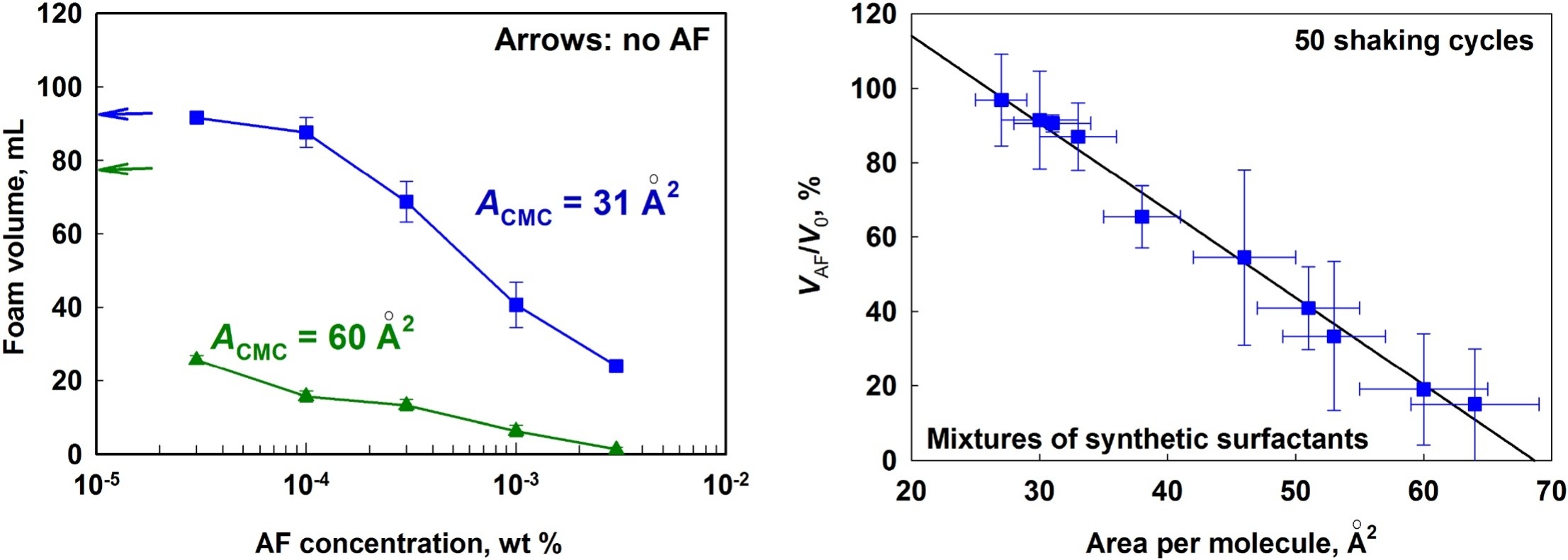
Role of surfactant adsorption and surface properties for the efficiency of PDMS-silica antifoams
We study how the composition of various surfactant mixtures affects the efficiency of mixed PDMS–silica antifoam in foamed surfactant solutions. First, systematic experiments are performed to characterize the surface and foam film properties of the studied surfactant solutions. The spreading, bridging and entry coefficients are calculated and the spreading ability of the antifoam is characterized by microscopy observations and by surface tension measurements. Next, the initial antifoam activity and the antifoam durability are characterized in foam tests. The obtained results reveal that the antifoam efficiency in solutions of low-molecular mass surfactants with low surface dilatational modulus depends strongly on the density (area-per-molecule) of the respective adsorption layer. The addition of nonionic surfactants, which increase the mean area-per-molecule in the mixed adsorption layer, enhances significantly the antifoam activity and durability. In contrast, the addition of surfactants, which decrease the mean area-per-molecule, suppresses the antifoam activity. Furthermore, we found that surfactant mixtures which form condensed adsorption layers on the solution surface suppress strongly the antifoam activity. As an extreme, the condensed adsorption layer formed from the natural surfactant Quillaja saponin suppresses the antifoam spreading even at highly positive spreading coefficient which results also in very poor AF efficiency. The obtained results rationalize in a coherent way the observed differences in the AF activity and durability in mixed solutions of various ionic, nonionic and zwitterionic surfactants.
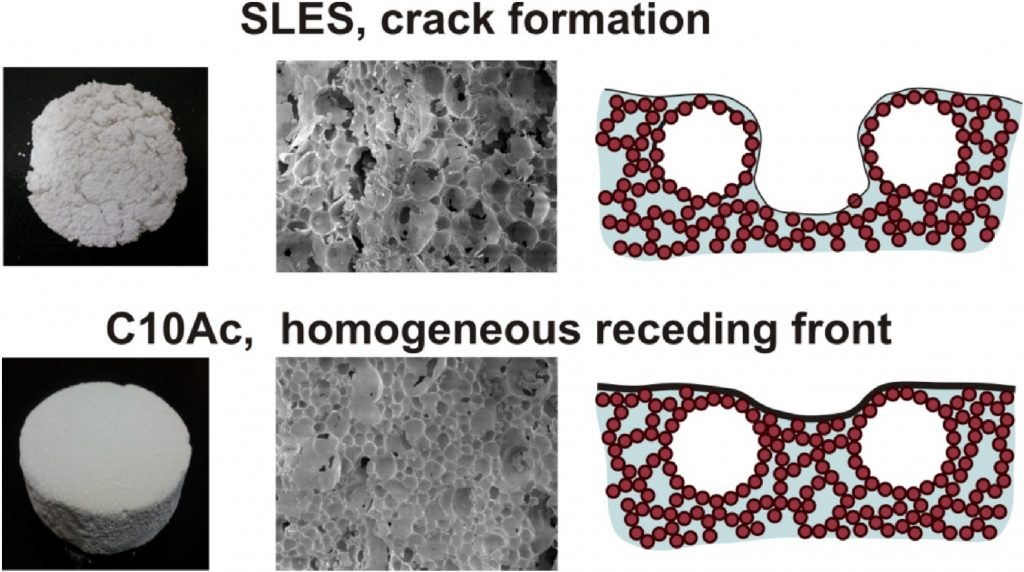
From Pickering foams to porous carbonate materials: Crack-free structuring in drying ceramics
Particle-stabilized foams have attracted considerable research interest, due to their long term stability (months to years) and the possibility to use them as precursors for production of porous materials with hierarchical porosity. In our previous study [Lesov et al., J. Colloid Interface Sci. 504 (2017) 48–57] we clarified the role of the rheological properties of the foamed suspensions and the type of foam film stabilization in the production of porous silica materials with low mass density and excellent insulating properties. In the current study we extend our approach to prepare lightweight carbonate ceramics with controlled density, shrinkage and good mechanical properties. To prepare the wet foam precursors, we tested a series of eight anionic surfactants which were previously reported to provide sufficient hydrophobization of CaCO3 particles and long-term stability of the liquid foams. From those surfactants, the medium-chain fatty acids led to crack-free porous materials with superior mechanical strength, compared to the conventional surfactants. We study the reasons for the formation of cracks in drying Pickering foams and, on this basis, propose optimal conditions for obtaining dry porous carbonate materials with required porosity. Mechanistic explanations are proposed for the main observed effects.