Marina Stoeva, Chemist
Interests
- Surfactans
- Micellar solutions
- Adsorption
- Solubilizations
- Detergency
Publications
Most recent publications
Role of electrolytes and co-surfactants on the rheological properties of sodium N-acyl sarcosinate solutions
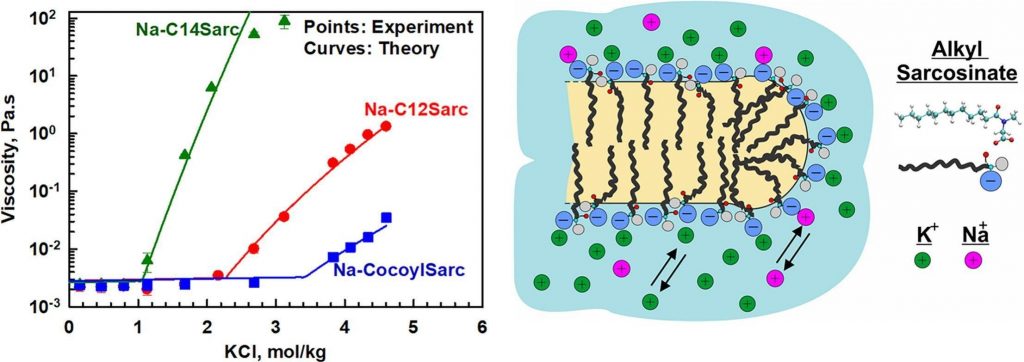
Alkyl sarcosinates are amino acid-based anionic surfactants commonly used as primary surfactants in sulfate-free personal care products. The major aim of this study is to identify the key factors influencing the rheological behaviour of sodium sarcosinate solutions and their mixtures with nonionic, zwitterionic, and cationic co-surfactants. To achieve this, we examined the effects of salt type and concentration for alkyl sarosinates with different chain lengths (dodecyl, tetradecyl, and cocoyl) across concentration range of 2–20 wt%. Experimental results reveal two distinct regions in the salt curve for the three studied sarcosinates. At low electrolyte concentrations, viscosity remains constant until reaching the critical electrolyte concentration, C1, beyond which viscosity increases logarithmically with salt concentration. Further electrolyte addition leads to phase separated solutions at the critical precipitation concentration, CTR. Both C1 and CTR decrease as the hydrocarbon chain length increases from dodecyl to tetradecyl. However, the presence of shorter chain molecules in cocoyl sarocisinate significantly increases both C1 and CTR due to the formation of spherical micelles. A theoretical expression for predicting viscosity dependence on salt concentration is derived and successfully applied to describe the experimental data. The adsorption energy of sodium and potassium to alkyl sacrosinate micelle surfaces is found to be much smaller than that to sodium lauroyl ether sulfate surfactants (1 vs. 3 kBT for Na+ and 0.8 vs. 3.8 kBT for K+). No significant effect of amphoteric co-surfactants, including cocoamidopropyl betaine, sulfobetaine, or decylamine oxide, is observed. NMR analysis confirms that cocoamidopropyl betaine and sodium dodecyl sarcosinate form mixed micelles that are structurally similar to sarcosinate micelles, as carboxyl groups remain exposed on the micelle surfaces in both cases. When using amine oxide and sulfobetaine, the increase in viscosity is attributed to the elongation of mixed micelles, though steric hindrance from side methyl groups limits their growth. The practical significance of this study lies in the finding that longer-chain alkyl sarcosinates (such as tetradecyl, as investigated here) can attain significantly higher viscosities at lower salt concentrations compared to shorter-chain analogs or surfactant mixtures. The scientific significance stems from the development of a theoretical model capable of predicting the viscosity of alkyl sarcosinate solutions across various surfactant and salt concentrations.