
Mariana P. Boneva, Ph.D.
Interests
- Detergency: Contact Angles and Wetting
- Particles at Fluid Interfaces: Electro-Dipping Forces
Bio
In 1996 she graduated the French Language High School, in Sliven, Bulgaria. In 2002 she got the degree M.Sc. in Chemistry, Faculty of Chemistry, Sofia University, Bulgaria, Major: “Substances of high and ultra high purity”. Since April 2001, she has been research associate in the Department of Chemical Engineering (DCE), Faculty of Chemistry, Sofia University, Bulgaria. Since February 2004 she has been PhD student. Her scientific interests are in the field of particles at fluid interfaces, incl. electrodipping force and electric-field induced capillary attraction; equilibrium of micelles and crystallites in carboxylate soap solutions, thin liquid films; drops and bubbles at interfaces, static and dynamic light scattering; electric conductivity, electrophoretic mobility and zeta-potential. So far, she has published 3 research articles, cited 36 times in the scientific literature.
Publications
Most recent publications
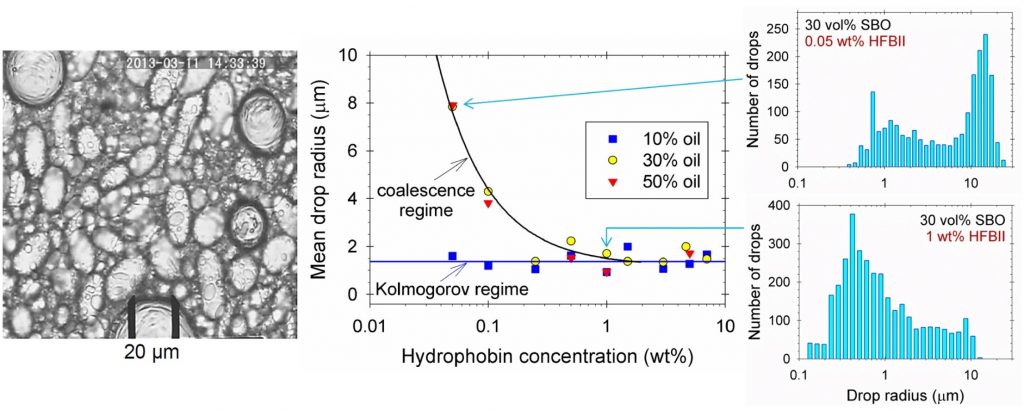
Limited coalescence and Ostwald ripening in emulsions stabilized by hydrophobin HFBII and milk proteins
Hydrophobins are proteins isolated from filamentous fungi, which are excellent foam stabilizers, unlike most of the proteins. In the present study, we demonstrate that hydrophobin HFBII can also serve as excellent emulsion stabilizer. The HFBII adsorption layers at the oil/water interface solidify similarly to those at the air/water interface. The thinning of aqueous films sandwiched between two oil phases ends with the formation of a 6 nm thick protein bilayer, just as in the case of foam films, which results in strong adhesive interactions between the emulsion drops. The drop-size distribution in hydrophobin stabilized oil-in-water emulsions is investigated at various protein concentrations and oil volume fractions. The data analysis indicates that the emulsification occurs in the Kolmogorov regime or in the regime of limited coalescence, depending on the experimental conditions. The emulsions with HFBII are very stable – no changes in the drop-size distributions are observed after storage for 50 days. However, these emulsions are unstable upon stirring, when they are subjected to the action of shear stresses. This instability can be removed by covering the drops with a second adsorption layer from a conventional protein, like β-lactoglobulin. The HFBII surface layer is able to suppress the Ostwald ripening in the case when the disperse phase is oil that exhibits a pronounced solubility in water. Hence, the hydrophobin can be used to stabilize microcapsules of fragrances, flavors, colors or preservatives due to its dense adsorption layers that block the transfer of oil molecules.
Coexistence of micelles and crystallites in solutions of potassium myristate: Soft matter vs. solid matter
Here, we investigate the coexistence of surfactant micelles and acid-soap crystallites in solutions of potassium myristate (n-tetradecanoate) to determine the micelle composition and charge, and the stoichiometry of the acid soaps. We carried out parallel pH, conductivity, and solubilization measurements, which indicate that micelles are present in the potassium myristate solutions at the higher concentrations, in contrast with the results for sodium myristate, where no micelles were detected at room temperature. Theoretical expressions describing the concentration dependences of conductivity and pH of the micellar carboxylate solutions are derived. Diagrams, showing the concentrations of all species in the solution, are constructed. The comparison of theory and experiment indicates that the undissociated fatty acid is incorporated in acid-soap crystallites, i.e. it behaves as initiator of crystallization. The rest of dissolved carboxylate forms micelles that are composed only of carboxylate anions and bound potassium counterions. Above 2-3 times the critical micellization concentration (CMC), the main mass of the carboxylate is in micellar form, despite the presence of coexisting acid-soap crystallites. Surface tension isotherms are obtained and interpreted on the basis of the results for the bulk composition. The adsorption layer is composed mostly of fatty acid and 1:1 acid-soap molecules. Not only the appearance of micelles, but also the change in the stoichiometry of the acid soaps in the solution leads to kinks, and even jumps, in the surface tension isotherm. The results for acid-soap stoichiometry have been confirmed by independent analysis of crystals collected from the solutions.
Attraction between particles at a liquid interface due to the interplay of gravity- and electric-field-induced interfacial deformations
In a previous study, we established that the attraction between electrically charged particles attached to a water/tetradecane interface is stronger than predicted on the basis of the gravity-induced lateral capillary force. Here, our goal is to explain this effect. The investigated particles are hydrophobized glass spheres of radii between 240 and 320 μm. Their weight is large enough to deform the liquid interface. The interfacial deformation is considerably greater for charged particles because of the electrodipping force that pushes the particles toward the water phase. By independent experiments with particles placed between two electrodes, we confirmed the presence of electric charges at the particle/tetradecane interface. The theoretical analysis shows that if the distribution of these surface charges is isotropic, the meniscus produced by the particle electric field decays too fast with distance and cannot explain the experimental observations. However, if the surface-charge distribution is anisotropic, it induces a saddle-shaped deformation in the liquid interface around each particle. This deformation, which is equivalent to a capillary quadrupole, decays relatively slow. Its interference with the gravity-induced isotropic meniscus around the other particle gives rise to a longrange attractive capillary force, F ∼ 1/L3 (L = interparticle distance). The obtained agreement between the experimental and theoretical curves, and the reasonable values of the parameters determined from the fits, indicate that the observed stronger attraction in the investigated system can be really explained as a hybrid interaction between gravity-induced “capillary charges” and electric-field-induced “capillary quadrupoles”.
Method for analysis of the composition of acid soaps by electrolytic conductivity measurements
Here, we propose a method for determining the stoichiometry of acid-soap crystallites. The method is based on dissolving the crystallites in water at an appropriate working temperature, followed by measurement of the electrolytic conductivity of the obtained solutions. The working temperature is chosen in such a way that the only precipitate in the solutions is that of carboxylic acid, whereas the carboxylate salt is dissociated, and its content in the dissolved crystals determines the solution’s conductivity. In the theoretical model for data interpretation, we took into account the dependence of the molar conductance on the ionic strength. The method was applied for determining the stoichiometry of acid-soap crystals collected from solutions of potassium myristate (tetradecanoate) at 25 °C. The crystals were dissolved in water at working temperature of 40 °C, at which the conductivity was measured. The stoichiometry of all samples determined in the present study coincides with that independently obtained by another method that is based on in situ pH measurements.
Effect of electric-field-induced capillary attraction on the motion of particles at an oil-water interface
Here, we investigate experimentally and theoretically the motion of spherical glass particles of radii 240-310 μm attached to a tetradecane-water interface. Pairs of particles, which are moving toward each other under the action of lateral capillary force, are observed by optical microscopy. The purpose is to check whether the particle electric charges influence the particle motion, and whether an electric-field-induced capillary attraction could be detected. The particles have been hydrophobized by using two different procedures, which allow one to prepare charged and uncharged particles. To quantify the hydrodynamic viscous effects, we developed a semiempirical quantitative approach, whose validity was verified by control experiments with uncharged particles. An appropriate trajectory function was defined, which should increase linearly with time if the particle motion is driven solely by the gravity-induced capillary force. The analysis of the experimental results evidences for the existence of an additional attraction between two like-charged particles at the oil-water interface. This attraction exceeds the direct electrostatic repulsion between the two particles and leads to a noticeable acceleration of their motion.

