
Lydia M. Dimitrova, Ph.D.
Interests
- Foams, antifoams
- Phase behavior of carboxylate solutions
- Equilibrium and dynamic surface tension
Publications
Most recent publications
Production and characterization of stable foams with fine bubbles from solutions of hydrophobin HFBII and its mixtures with other proteins
Hydrophobins are proteins that are excellent foam stabilizers. We investigated the effects of pH and addition of other proteins on the foaminess, bubble size, and stability of foams from aqueous solutions of the protein HFBII hydrophobin. The produced stable foams have bubbles of radii smaller than 40 μm that obey the lognormal distribution. The overrun of most foams is in the range from 5 to 8, which indicates a good foaminess. The foam longevity is characterized by the time dependences of the foam volume and weight. A combined quantitative criterion for stability, the degree of foam conservation, is proposed. The produced foams are stable for at least 12–17 days. The high foam stability can be explained with the formation of dense hydrophobin adsorption layers, which are impermeable to gas transfer and block the Ostwald ripening (foam disproportionation). In addition, the population of small bubbles formed in the HFBII solutions blocks the drainage of water through the Plateau borders in the foam. The variation of pH does not essentially affect the foaminess and foam stability. The addition of “regular” proteins, such as beta-lactoglobulin, ovalbumin and bovine serum albumin, to the HFBII solutions does not deteriorate the quality and stability of the produced foams up to 94% weight fraction of the added protein. The results and conclusions from the present study could be useful for the applications of hydrophobins as foam stabilizers.
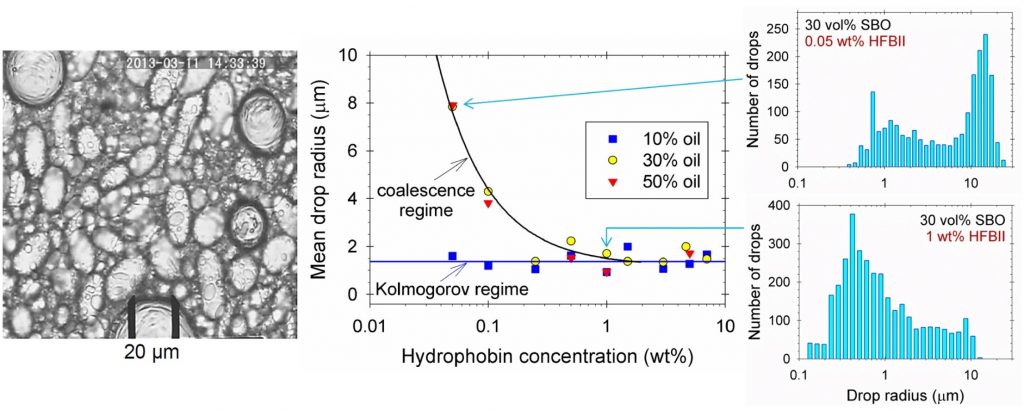
Limited coalescence and Ostwald ripening in emulsions stabilized by hydrophobin HFBII and milk proteins
Hydrophobins are proteins isolated from filamentous fungi, which are excellent foam stabilizers, unlike most of the proteins. In the present study, we demonstrate that hydrophobin HFBII can also serve as excellent emulsion stabilizer. The HFBII adsorption layers at the oil/water interface solidify similarly to those at the air/water interface. The thinning of aqueous films sandwiched between two oil phases ends with the formation of a 6 nm thick protein bilayer, just as in the case of foam films, which results in strong adhesive interactions between the emulsion drops. The drop-size distribution in hydrophobin stabilized oil-in-water emulsions is investigated at various protein concentrations and oil volume fractions. The data analysis indicates that the emulsification occurs in the Kolmogorov regime or in the regime of limited coalescence, depending on the experimental conditions. The emulsions with HFBII are very stable – no changes in the drop-size distributions are observed after storage for 50 days. However, these emulsions are unstable upon stirring, when they are subjected to the action of shear stresses. This instability can be removed by covering the drops with a second adsorption layer from a conventional protein, like β-lactoglobulin. The HFBII surface layer is able to suppress the Ostwald ripening in the case when the disperse phase is oil that exhibits a pronounced solubility in water. Hence, the hydrophobin can be used to stabilize microcapsules of fragrances, flavors, colors or preservatives due to its dense adsorption layers that block the transfer of oil molecules.
Impact of the surfactant structure on the foaming/defoaming performance of nonionic block copolymers in Na caseinate solutions
Systematic experimental study of the physico-chemical factors controlling the foaming and defoaming performance of several Pluronic nonionic block copolymers is performed in solutions of sodium caseinate. Dynamic light scattering measurements and microscopic observations confirm that a significant defoaming is observed above the cloud point of the nonionics, which is almost independent on the polymer structure. However significant differences are observed in the foaming below the cloud point, which do correlate with the polymer structure. Dynamic surface tension measurements allow more detailed analysis of the mechanism of polymer and protein adsorption, and of the role of the individual components for the overall stabilization or destabilization of the foams.

