Lucie Delforce
Interests
- Adsorbed layers and surface properties
- Foams, Emulsions, Microemulsions
Bio
Publications
Most recent publications
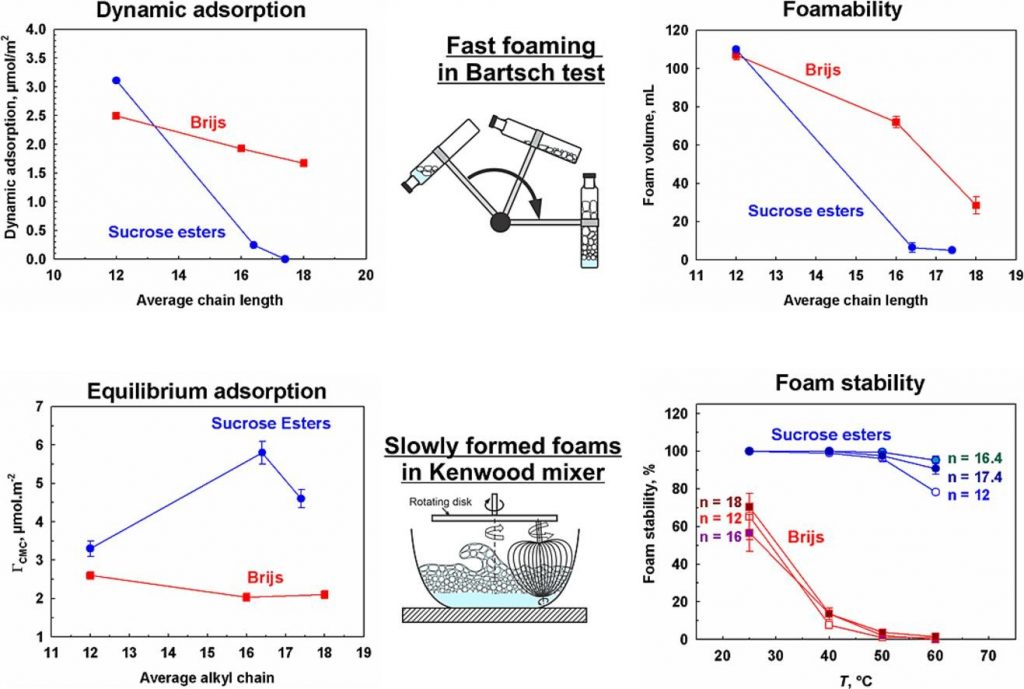
Alkyl sucrose esters vs. Brijs: How chain length and temperature impact surface and foam properties
The primary objective of this study is to determine the similarities and differences in the surface, film, and foam properties of alkyl sucrose esters (SEs) with high monoester content (≥ 70 %) and polyoxyethylene alkyl ethers (Brijs) with a high number of ethoxy groups (≥ 20) in their head group. Experiments were conducted using surfactant molecules with alkyl chain lengths of 12, 16, and 18 carbon atoms at concentrations between 0.01 and 1 wt%, within a temperature range of 25 °C to 60 °C.
The lag time for surfactant adsorption increased with surfactant chain length, decreased with temperature, and significantly decreased with surfactant concentration for both types of studied surfactants. However, increasing the chain length from 12 to 18 carbon atoms led to a 10-fold increase in lag time for Brijs and more than a 600-fold increase for SEs. This effect rendered longer-chain SEs incapable of forming voluminous foam in Bartsch test and led to pronounced coalescence between the bubbles after their separation from the sparger in the foam rise method, resulting in foams with very large bubbles, which exhibited lower stability. The utilization of a Kenwood mixer for foam generation provided sufficient time for longer-chain SE molecules to adsorb on the bubble surfaces and to produce voluminous foams with small bubbles, which remained stable even at 60 °C. In contrast, foams generated from Brijs solutions are very unstable at 60 °C. The long-standing stability of SEs foam was attributed to the formation of mixed mono- and diesters adsorption layers on the bubble surfaces.
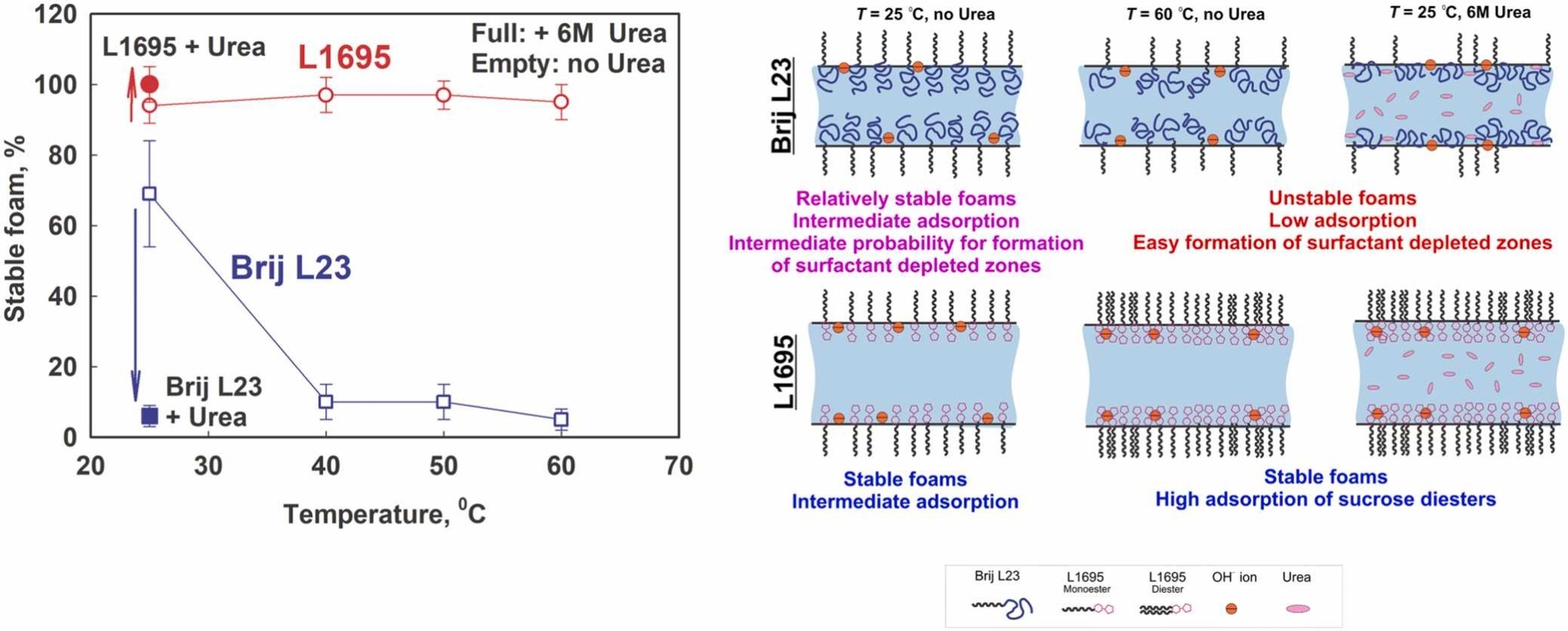
Role of temperature and urea for surface and foam properties of nonionic surfactants with dodecyl alkyl chain
The surface, film and foam properties of two nonionic surfactants, dodecyl sucrose ester (L1695) and dodecyl polyoxyethylene ether (Brij L23), were studied at four different temperatures between 25 and 60 °C and three surfactant concentrations (0.01%, 0.1%, and 1% wt.). The impact of 6 M urea was also assessed to determine the role of hydrogen bonds for the observed trends. The foams were generated using two methods: the fast foaming method (Bartsch test), producing foams with smaller bubbles, and the slow foaming method (foam rise method), yielding foams with bigger bubbles. For Brij L23, an increase in temperature resulted in a decrease in the critical micellar concentration (CMC), reduced surfactant adsorption on the air-water interface, weakened electrostatic repulsion between the foam film surfaces and significantly decreased foam stability. For L1695, an increase in temperature increased surfactant adsorption, maximized the foamability at 40 °C, and did not affect the foam stability for foams with small bubbles. However, the temperature increase leads to decreased stability at low concentrations for foams with bigger bubbles. The addition of 6 M urea, resulted in increased adsorption without any effect on foam stability for L1695, whereas it decreased the adsorption and foam stability for Brij L23. The comparison of relative foamability vs. dynamic surface coverage revealed that a lower threshold surface coverage is required to increase the foamability of L1695 (≈ 80%) compared to Brij L23 (≈ 95%). This difference is explained by the action of weak electrostatic repulsion and the adsorption of sucrose diesters on the bubble surfaces when L1695 surfactant is used. The higher stability of L1695 foams under all studied conditions is attributed to the formation of a denser adsorption layer due to the adsorption of sucrose diesters. The diesters prevent the formation of weak spots within the foam films, even at high temperatures. This work contributes to the advancement of the foam field by demonstrating that a mixture of sucrose mono and diester surfactants can be highly effective in forming stable foams at higher temperatures and in the presence of urea. Both factors (higher temperature and 6 M urea) have negative effect on Brij L23 foams, while they have no significant effect on L1695 foams with smaller bubbles.