
Prof. Krastanka G. Marinova, Ph.D.
Interests
- Cosmetics and Homecare Formulations
- Surfactant Adsorption and Interfacial Rheology
- Foams, Antifoams and Detergency
- Thin Liquid Films
Bio
M.Sc. in Physics (1991); Ph.D. in Physical Chemistry (2002); Assistant Professor (2003); Assoc. Professor (2010), Professor in Physical Chemistry (2025) in the Faculty of Chemistry and Pharmacy, University of Sofia “St. Kliment Ohridski”, Bulgaria. Vice Dean of the Faculty of Chemistry and Pharmacy (2012-2019). Coordinator of the Master’s program “Cosmetic Science and Homecare Formulations”. Member (from 2016) and elected President (April 2017) of the Bulgarian Association of Cosmetologists. She has been a visiting researcher in the Department of Chemical Engineering, University of Patras, (Patras, Greece, 1993) and in the Research Center of the company Rhodia Silicones Europe (Lyon, France, 1998). Her expertise includes experimental techniques for studying liquid interfaces (kinetics of adsorption and interfacial rheology), thin liquid films and foams, electrophoretic phenomena, computer methods for data processing and analysis, formulation of dispersions for cosmetics, personal and home care. Her main research interests are in the areas of kinetics of surfactant adsorption and interfacial rheology, incl. development of new experimental methods (in collaboration with Krüss GmbH, Germany); foams and antifoams; surface forces and stability of thin films, formulation of dispersions for cosmetics, personal and home care. She was the recipient of the award “Best Young Scientist” for 1999 of the University of Sofia Foundation “St. Kliment Ohridski”. So far, she has published more than 45 research and review articles, 1 patent, 2 patent applications, cited more than 1900 times in the scientific literature, h=20.
Publications
Featured publications
New surfactant mixtures for fine foams with slowed drainage
We form and investigate foams stabilized by a triple surfactant mixture containing a nonionic alkyl polyglucoside (APG) in addition to the combination of ionic sodium lauryl-dioxyethylene sulphate (SLES) and zwitterionic cocamidopropyl betaine (CAPB) surfactants. APG improves the surfactants compatibility at alkaline pH. The addition of a readily biodegradable chelating agent methylglycinediacetic acid (MGDA) in the mixture contributes further for the excellent performance even in very hard water. Foam properties are analyzed and compared to those of the single components and to the binary mixture without APG. Foam drainage is successfully controlled by introducing additives suitable for the alkaline conditions: fatty alcohol and/or hydrophobically modified starch. Systematic model experiments are performed to characterize the surface tension and dilatational rheology, and thin films drainage. Slowed foam and thin films drainage is confirmed to correlate with the increased surface visco-elasticity in the presence of fatty alcohols. Temperature impact on the surface properties is used for fine tuning of the foam drainage.
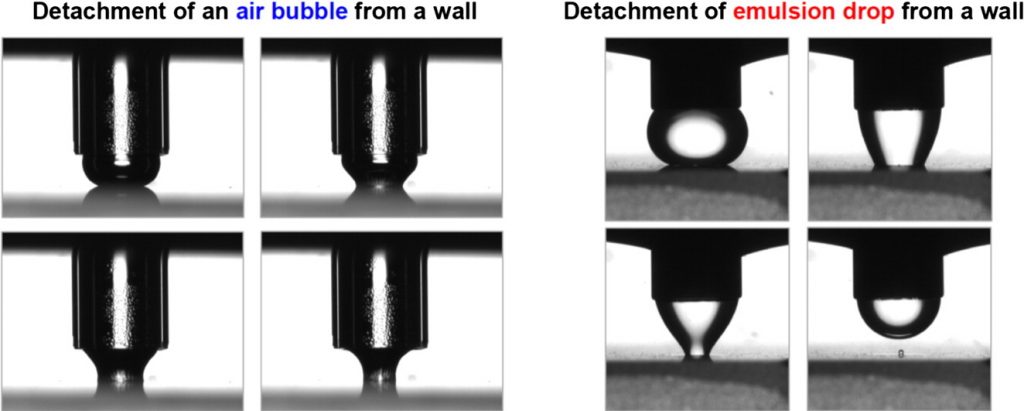
Adhesion of bubbles and drops to solid surfaces, and anisotropic surface tensions studied by capillary meniscus dynamometry
Here, we review the principle and applications of two recently developed methods: the capillary meniscus dynamometry (CMD) for measuring the surface tension of bubbles/drops, and the capillary bridge dynamometry (CBD) for quantifying the bubble/drop adhesion to solid surfaces. Both methods are based on a new data analysis protocol, which allows one to decouple the two components of non-isotropic surface tension. For an axisymmetric non-fluid interface (e.g. bubble or drop covered by a protein adsorption layer with shear elasticity), the CMD determines the two different components of the anisotropic surface tension, σs and σφ, which are acting along the “meridians” and “parallels”, and vary throughout the interface. The method uses data for the instantaneous bubble (drop) profile and capillary pressure, but the procedure for data processing is essentially different from that of the conventional drop shape analysis (DSA) method. In the case of bubble or drop pressed against a substrate, which forms a capillary bridge, the CBD method allows one to determine also the capillary-bridge force for both isotropic (fluid) and anisotropic (solidified) adsorption layers. The experiments on bubble (drop) detachment from the substrate show the existence of a maximal pulling force, Fmax, that can be resisted by an adherent fluid particle. Fmax can be used to quantify the strength of adhesion of bubbles and drops to solid surfaces. Its value is determined by a competition of attractive transversal tension and repulsive disjoining pressure forces. The greatest Fmax values have been measured for bubbles adherent to glass substrates in pea-protein solutions. The bubble/wall adhesion is lower in solutions containing the protein HFBII hydrophobin, which could be explained with the effect of sandwiched protein aggregates. The applicability of the CBD method to emulsion systems is illustrated by experiments with soybean-oil drops adherent to hydrophilic and hydrophobic substrates in egg yolk solutions. The results reveal how the interfacial rigidity, as well as the bubble/wall and drop/wall adhesion forces, can be quantified and controlled in relation to optimizing the properties of foams and emulsions.
Co-adsorption of the proteins ß-casein and BSA in relation to the stability of thin liquid films and foams
Most recent publications
Disperse systems and rheology in clean technologies – lab 6.2 capabilities and developments
Laboratory 6.2 “Disperse systems and rheology in clean technologies” within WP 6 “Nano-structured materials and disperse systems in clean technologies” aims at the development of new systems for clean technologies and nanotechnologies in various scientific and technological areas: new cleaning agents for hard surfaces; encapsulation and controlled release of reagents; wetting and dewetting processes. The main objectives are to conduct experimental and theoretical research and development of new dispersed systems through the inclusion of new raw materials and innovative materials, and to develop and apply theoretical models of the interactions and stability of complex dispersed systems. High scientific output of Lab 6.2 is manifested via 21 scientific publications in the fields of Dispersion Chemistry, Chemical and Materials Sciences for the period 2018 – 2024 (8 articles in Q1 journals, 6 in Q2, and 7 materials in Q4). Optimized compositions of cleaning formulations for hard surfaces with sulfonated methyl esters have been obtained [1]. New theoretical models for the interactions that determine the structures in micellar systems have been developed and applied [2]. New phenomena such as vortexing [3] and nanoemulsification [4] have been described. New methods have been proposed for obtaining emulsions and foams from triglyceride phases [5]. The role of the components and compositions for the rheology [6], wetting, and cleaning [7] has been demonstrated.
Multiconnected micellar phases for cleaning applications
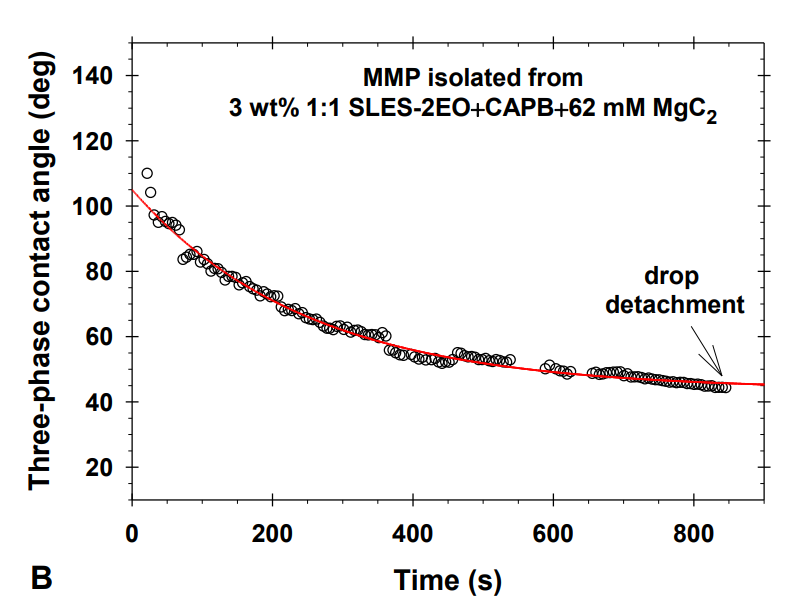
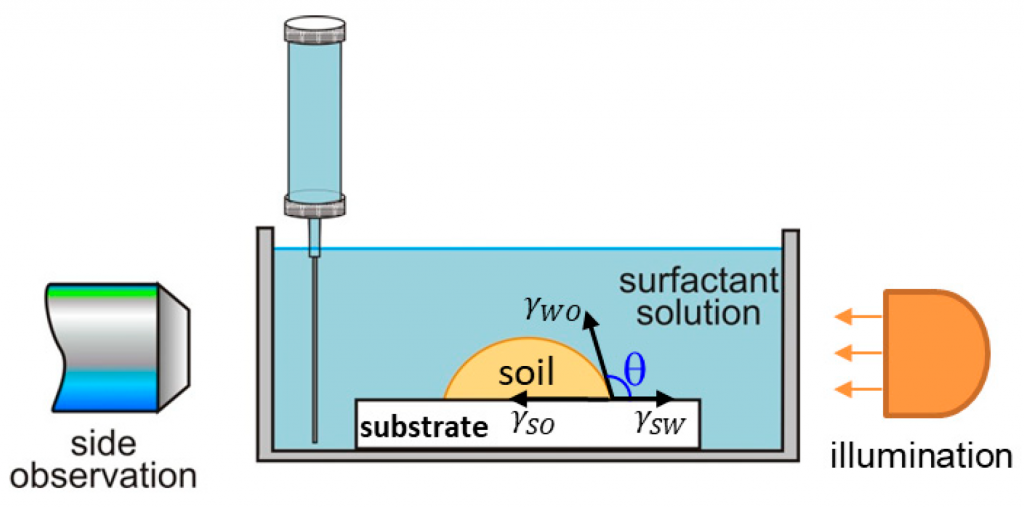
The research paper explores the ability of isolated multiconnected micellar phases (MMP) to remove different soils (dimethicone, sebum oil, and makeup formulations) from artificial skin. The MMP is isolated from 3 wt% 1:1 sodium lauryl ether sulfate with 2 ethylene oxide group + cocamidopropyl betaine in the presence of 62 mM MgCl2. The direct observations of soil removal demonstrate the efficacy of the MMP by two physicochemical mechanisms. For dimethicone soils, the roll-up mechanism is observed, i.e. shrinkages of the drop contact areas and decrease of the three-phase contact angles due to the changes in the interfacial tension and the surface energy. For sebum soils, the spontaneous emulsification mechanism takes place because of the mixing of the fatty acids present in the sebum with the surfactants and forming complex amphiphilic structures. For makeup formulations, the new approach based on the software image analysis by means of Image J is developed to account for the whole soiled area and to increase the precision of the quantitative cleaning characterization. The obtained results show that the bicontinuous phase successfully removes dimethicone, sebum oil, and foundation soils from the artificial skin and could have potential application in the design of new personal, home and industrial care products.
Cleansing mechanisms and efficacy on artificial skin
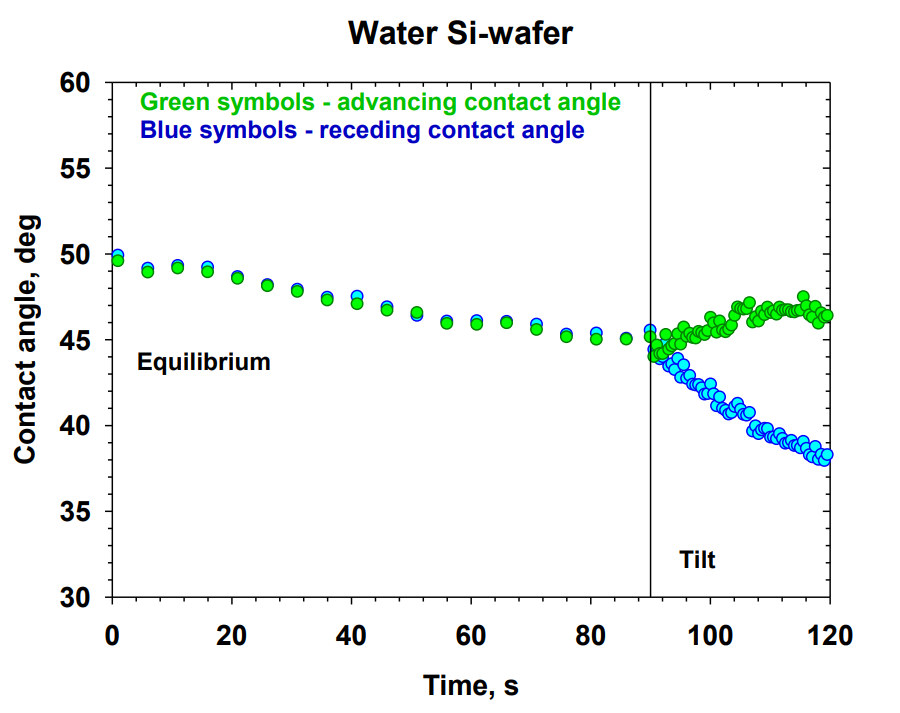
A systematic study on the mechanisms of cleansing artificial skin by solutions of widely used in personal care surfactants disodium laureth sulfosuccinate (DSLSS), sodium laureth sulfate (SLES), sodium dodecyl sulfate (SDS), dodecyl trimethyl ammonium bromide (DTAB), and coco glucoside (CG), is presented. The systematic characterization of soil removal from artificial skin revealed two primary cleansing mechanisms: emulsification and roll-up. Emulsification occurs in systems with very low interfacial tension, such as sebum in SLES solutions, while dimethicone soil was only removed by roll-up. The roll-up effectiveness depends on the surfactant’s interfacial activity and its adsorption on the soiled surface. Thus, the strong adsorption of DTAB on the skin leads to dimethicone roll-up at a relatively high interfacial tension of 11 mN/m. The anionic and nonionic surfactants adsorbed less at the artificial skin surface, and the oil/water interfacial tension value lowering below 5 mN/m is necessary for the roll-up to occur. Nonionic CG removed dimethicone at a lower concentration than ionic surfactants. Combining CG with ionic surfactants improved cleaning at lower total concentrations. Surfactant mixtures are used to formulate simple cleansing formulations, whose performance is also investigated by the developed in vitro approach. The results obtained allow for a good rating of the formulations, which correlates well with the performance of the surfactant mixtures and their interfacial activity.
Spontaneous bubble growth inside high-saturation-vapor-pressure and high-air-solubility liquids and emulsion droplets
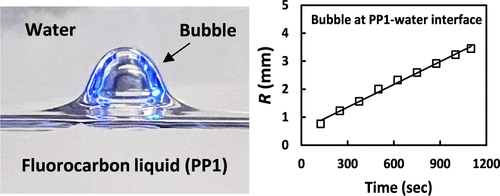
Spontaneous bubble growths in liquids are usually triggered by rapid changes in pressure or temperature that can lead to liquid gas supersaturation. Here, we report alternative scenarios of the spontaneous growths of bubbles inside a high-saturation-vapor-pressure and high-air-solubility perfluorocarbon liquid (PP1) that were observed under ambient quiescent conditions. First, we investigate spontaneous bubble growth inside the single PP1 phase, which was left to evaporate freely. The bubble growth is explained by the difference in the PP1 vapor pressure inside the bubble and that above the freely evaporating PP1 interface. Next, we study the bubble growth inside the liquid PP1 covered with a layer of a second air-saturated immiscible liquid: low-air-solubility water or higher-air-solubility ethanol. In both cases, the bubble growth rates were accelerated, indicating mass transfer of air from the water or ethanol phases to the PP1 phase. The bubble growth rates significantly increase for bubbles trapped at the PP1–water or PP1–ethanol interfaces due to faster air diffusion through the thin PP1 liquid films separating the bubbles from the upper phases. Finally, we consider the case of bubbles inside millimeter-sized PP1 emulsion droplets in water or ethanol. The bubble growth inside the droplet leads to an increase in the PP1 droplet’s effective buoyancy and to the detachment of the droplets from the substrate. The observed bubble growth rate in the case of emulsion droplets was much faster for PP1 droplets in ethanol than for PP1 droplets in water (minutes vs hours). The underlying physical mechanism of the increase of bubble volumes is the spontaneous mass transfer of both air and PP1 vapor to the bubbles produced by a colloidal diffusion pump effect.
Investigation of the detergency properties of mixtures of biocides and nonionic surfactants using a new simplified hard surface cleaning method
The present study explores the cleaning efficacy of a set of nonionic surfactants (linear ethoxylated alcohol, secondary ethoxylated alcohols with 5, 7, and 9 ethoxy groups, glycoside surfactants, polyglycerol surfactants, and an ethoxylated sorbitan monolaurate) combined with cationic biocides—alkyl quaternary ammonium salts. A simple hard surface cleaning methodology was applied, which was shown to discriminate well between poor and good cleaning formulations. In addition to cleaning efficacy, surface aesthetics such as gloss and haze were evaluated together to assess surface streaking caused by a residual surfactant layer. The haziness determination turned out to be the key feature revealing the complex cleaning performance of multi-component products.


