Prof. Krassimir D. Danov, Ph.D., D.Sc.
Corresponding Member of Bulgarian Acad. Sci.
Head of the Laboratory of Complex Fluids
Interests
- Hydrodynamics of Liquid Films, Drops and Bubbles
- Interfacial Rheology and Bulk Rheology of Dispersions
- Thermodynamics and Kinetics of Surfactant Adsorption
- Kinetics of Coagulation and Flocculation
- Instabilities and Critical Thickness of Liquid Films
Bio
M.Sc. Mechanics and Mathematics (1979); Ph.D. Mathematics (1985); Associate Professor (1997); D.Sc. (2001), Full Professor of Mathematical Modeling and Applied Mathematics (2005) – Department of Chemical Engineering (DCE), Faculty of Chemistry, Sofia University, Bulgaria. Five times, he has been visiting professor in the Institute of Fluid Mechanics, University of Erlangen – Nuremberg, Germany. He was also senior researcher in the CRPP, Bordeaux, France, 1999, and in the Laboratory of Ultrastructure Research, NIPS, Okazaki, Japan, 2001. His research interests are in the area of the hydrodynamics of liquid films, drops and bubbles; thermodynamics and kinetics of surfactant adsorption, incl. micellar surfactant solutions; interfacial rheology and bulk rheology of suspensions; evaporation, hydrodynamic instabilities and critical thickness of liquid films; dynamics of flocculation and coalescence in emulsions; electrostatic and hydrodynamic interactions of colloidal particles at fluid phase boundaries and in thin liquid films. So far, he has published 217 research and review papers (cited 5471 times in the scientific literature, h-index 44, Scopus), and has given 296 presentations at international conferences, including 76 invited lectures and 115 lectures. He was awarded the F. W. Bessel Prize of the Humboldt Foundation (Germany) in 2002 and the Blue Ribbon Medal of the Sofia University “St. Kliment Ohridski” for significant achievements in science (2016). He is recipient of the highest national award for scientific achievements “Pythagoras” by the Bulgarian ministry of education and science (2019).
Publications
Most recent publications
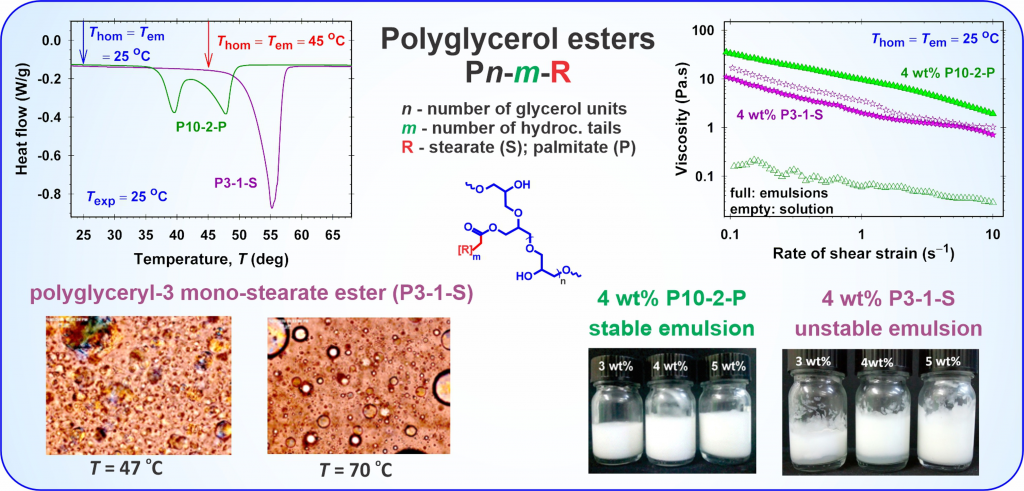
Aqueous Solutions of Oil-Soluble Polyglycerol Esters: Structuring and Emulsifying Abilities
The polyglycerol esters (PGEs) of fatty acids have a wide range of HLB values and applications in diverse industries, such as pharmaceuticals and cosmetics. While the physicochemical properties of oil-soluble PGEs dissolved in oil phases are well studied in the literature, there is no information on their structuring in aqueous phases and emulsifying abilities. We combined rheological and differential scanning calorimetry (DSC) measurements and microscopy observations to characterize the dependence of oil-soluble PGE structuring in aqueous phases on the PGE concentration, the temperature of solution homogenization, and the PGE molecular structure. Excellent correlations between the considerable changes in solution viscosity and the temperatures of the two endo- and exothermic peaks in the DSC thermograms are observed. Single-tail PGE molecules, which have a higher number of polyglycerol units, are better organized in networks, and the viscosity of their aqueous solutions is higher compared to that of the respective double-tail PGE molecules. PGEs exhibit good emulsifying ability and the viscosity of the produced emulsions at room temperature can differ by orders of magnitudes depending on the temperature of emulsification. The reported properties of oil-soluble PGEs could be of interest for increasing the range of their applicability in practice.
On the rheology of linear wormlike micellar solutions
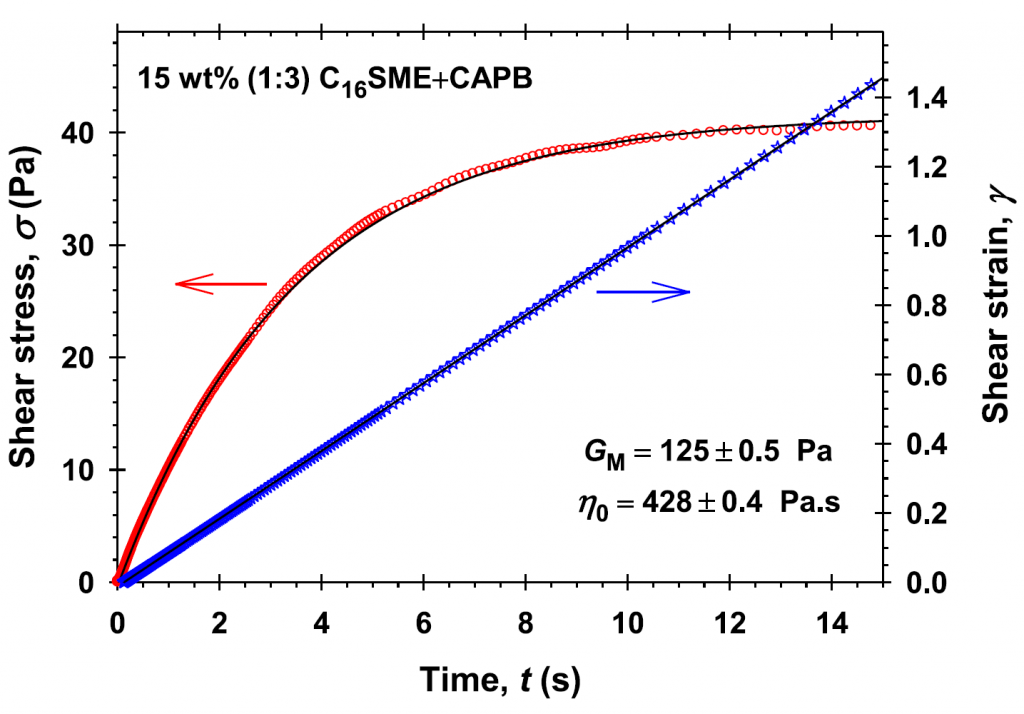
The shear rheology of linear wormlike micellar solutions (WMSs) is described by both Poisson renewal (PRM)and shuffling (SFM) models with different values of the model parameters. For low shear strains and rates ofstrains, the micellar solutions behave as a Maxwellian body with constant elasticity and viscosity. The excellentdescription of experimental data in the literature using PRM or SFM suggests that both models predict identicaldependencies of the dynamic storage and loss moduli on the frequency of oscillations. It is shown in the literature, that the PRM becomes equivalent to the SFM, when the breaking time is constant, τbr, and the characteristic reptation time, τrep, is equal to π2 τd0, where τd0 is the reptation time evaluated with respect to the average length of the chain. Three independent rheological tests (apparent viscosity vs shear rate, stress vs strain at constant shear rates, strain oscillations at low amplitudes and different frequencies) are applied to low, medium, and high zero-shear viscosity WMSs to obtain the PRM and SFM model parameters (elasticity, viscosity, relaxation, breaking, and reptation times). The known closed-form analytical expression for the Laplace image of the stress relaxation function and the respective infinite series for the complex modulus give possibility for the reported here precise systematic calculations of the storage and elastic moduli, the crossover frequency, and the elasticity for all values of ζbr = τbr/τrep ≤ 100. The predictions of the PRM length-dependent breaking-time versions are indistinguishable from those of the SFM for the obtained universal dependencies of the characteristic time, τB0, on ζbr. The applicability of the Vasquez–Cook–McKinley and the single-mode Oldroyd 8-constant models to describe the rheological behavior of WMSs is tested. The theoretical findings and conclusions are confirmed experimentally and illustrate the self-consistency of the used rheological regimes.
Disperse systems and rheology in clean technologies – lab 6.2 capabilities and developments
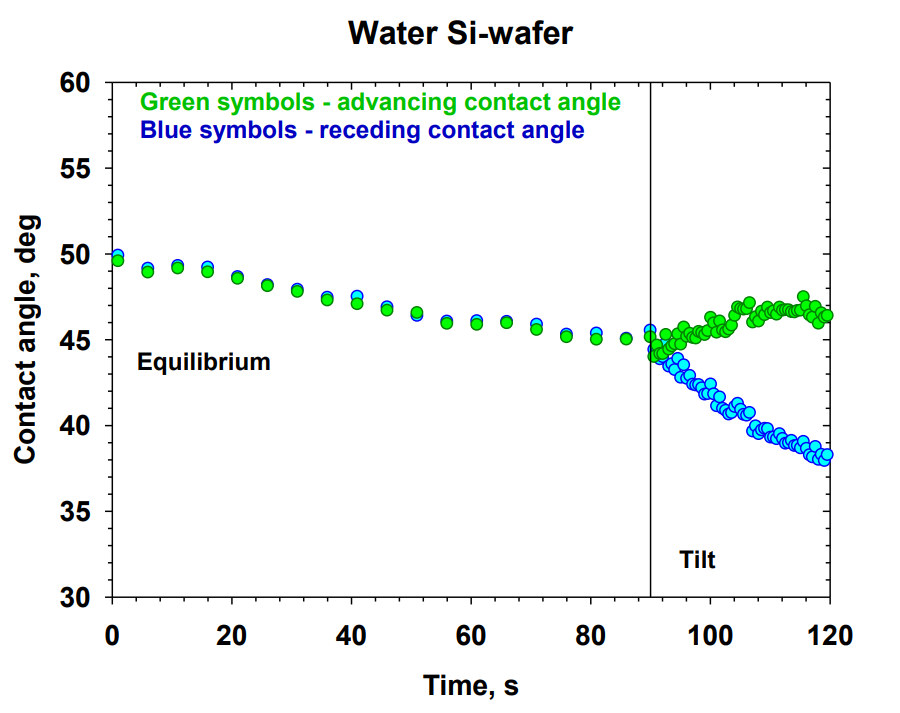
Laboratory 6.2 “Disperse systems and rheology in clean technologies” within WP 6 “Nano-structured materials and disperse systems in clean technologies” aims at the development of new systems for clean technologies and nanotechnologies in various scientific and technological areas: new cleaning agents for hard surfaces; encapsulation and controlled release of reagents; wetting and dewetting processes. The main objectives are to conduct experimental and theoretical research and development of new dispersed systems through the inclusion of new raw materials and innovative materials, and to develop and apply theoretical models of the interactions and stability of complex dispersed systems. High scientific output of Lab 6.2 is manifested via 21 scientific publications in the fields of Dispersion Chemistry, Chemical and Materials Sciences for the period 2018 – 2024 (8 articles in Q1 journals, 6 in Q2, and 7 materials in Q4). Optimized compositions of cleaning formulations for hard surfaces with sulfonated methyl esters have been obtained [1]. New theoretical models for the interactions that determine the structures in micellar systems have been developed and applied [2]. New phenomena such as vortexing [3] and nanoemulsification [4] have been described. New methods have been proposed for obtaining emulsions and foams from triglyceride phases [5]. The role of the components and compositions for the rheology [6], wetting, and cleaning [7] has been demonstrated.
Multiconnected micellar phases for cleaning applications
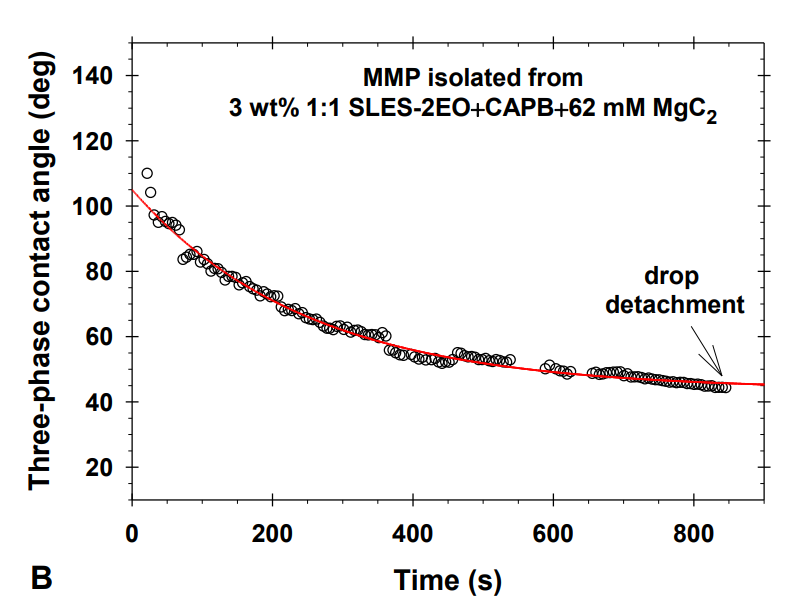
The research paper explores the ability of isolated multiconnected micellar phases (MMP) to remove different soils (dimethicone, sebum oil, and makeup formulations) from artificial skin. The MMP is isolated from 3 wt% 1:1 sodium lauryl ether sulfate with 2 ethylene oxide group + cocamidopropyl betaine in the presence of 62 mM MgCl2. The direct observations of soil removal demonstrate the efficacy of the MMP by two physicochemical mechanisms. For dimethicone soils, the roll-up mechanism is observed, i.e. shrinkages of the drop contact areas and decrease of the three-phase contact angles due to the changes in the interfacial tension and the surface energy. For sebum soils, the spontaneous emulsification mechanism takes place because of the mixing of the fatty acids present in the sebum with the surfactants and forming complex amphiphilic structures. For makeup formulations, the new approach based on the software image analysis by means of Image J is developed to account for the whole soiled area and to increase the precision of the quantitative cleaning characterization. The obtained results show that the bicontinuous phase successfully removes dimethicone, sebum oil, and foundation soils from the artificial skin and could have potential application in the design of new personal, home and industrial care products.
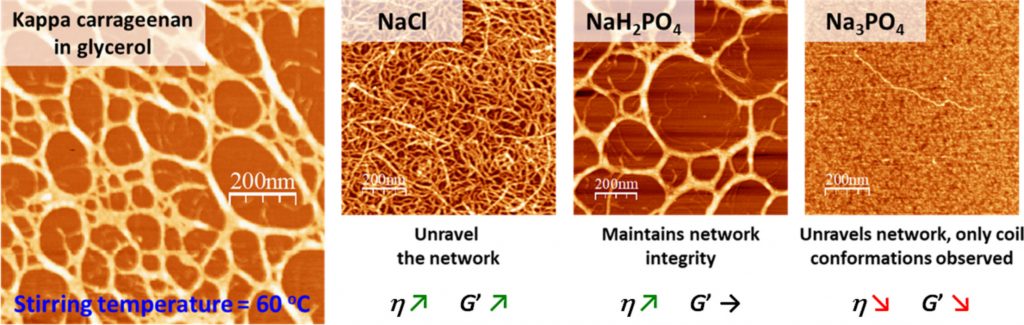
Conformational and rheological behavior of kappa carrageenan in glycerol: Effects of sodium salts and preparation temperature
Kappa carrageenan (KC), a sulfated polysaccharide derived from red seaweed, exhibits distinct gelation properties that are influenced by ionic strength and thermal conditions. While its behavior in aqueous media is well-established, understanding KC’s gelation mechanisms in non-aqueous solvents (like glycerol) remains limited. This study investigates the conformational and rheological properties of kappa carrageenan in glycerol, focusing on the effects of sodium salts (NaCl, NaH2PO4, Na3PO4) at varying concentrations and preparation temperatures (60 °C and 80 °C). Rheological measurements reveal distinct viscosity trends influenced by salt type and temperature, highlighting the interplay between ionic interactions and KC’s conformational transitions. Phosphate salts significantly enhance network elasticity and stability, especially at intermediate concentrations, whereas NaCl induces weaker, viscosity-dominated structures. Atomic force microscopy imaging provides complementary nanoscale insights, showcasing salt-specific structural transitions from looped to branched networks, alongside a temperature-dependent helix-to-coil transformation. These results illustrate how the precisely tuning ionic conditions and the preparation temperatures in glycerol media can effectively modulate KC’s structure and viscoelastic properties. This deeper understanding facilitates targeted design and optimization of carrageenan-based materials across food, pharmaceutical, cosmetic, and biotechnological applications.