
Ivan U. Vakarelski, Ph.D.
Interests
- Bubble and Droplet interactions
- Superhydrophobicity
- Leidenfrost effect
- Hydrodynamic drag
Bio
Dr. Vakarelski obtained his M.S. in Physics from Sofia University (1990) and his Ph.D. in Chemical Engineering from Kyoto University, Japan (2001). He has held research positions at the University of Florida (USA), Kyoto University (Japan), the University of Melbourne (Australia), and the Institute of Chemical and Engineering Sciences (Singapore). From 2010 to 2021, he was a Senior Research Scientist at King Abdullah University of Science and Technology (KAUST), Saudi Arabia.
Since 2023 he rejoined the Department of Chemical and Pharmaceutical Engineering at Sofia University as Senior Researcher (R4) while maintaining active collaborations with KAUST. His research interests include microparticle, bubble and droplet interactions investigated by AFM; nanoparticle self-assembly via evaporative lithography; interface mobility effects on bubbles and droplets dynamics; superhydrophobicity and the Leidenfrost effect; and lubricating gas layers for drag reduction and energy saving.
Dr. Vakarelski has published over 80 research articles, including lead-author contributions in Nature (1), Science Advances (2), PNAS (1), and Physical Review Letters (4). His work has been cited >3,800 times (h-index = 36).
Publications
Most recent publications
Self-propelling Leidenfrost ratchet boats
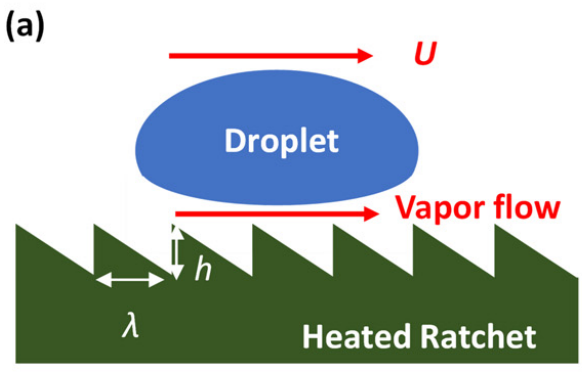
A small liquid droplet placed on an asymmetric ratchet surface heated above the Leidenfrost temperature is known to self-propel. Here, we examine an alternative configuration: a heated macroscopic alumina boat (14 cm long) decorated with asymmetric ratchets and floated in volatile perfluorocarbon liquid. Boats with 1 mm pitch ratchets, heated to 280 C, showed robust self-propulsion in the expected direction, reaching several centimeters per second and traveling more than one meter. An impulse–momentum balance scaling, derived from Leidenfrost droplet propulsion, predicts boat velocities of the same order but somewhat below experiments, suggesting enhanced efficiency of vapor flow in contact with bulk liquid. Propulsion efficiency depends strongly on ratchet geometry: for small pitches, bottom and sidewall ratchets act synergistically, while at larger pitches, sidewall ratchets reduce thrust by redirecting vapor. Herringbone-patterned ratchets further enhanced propulsion. Towing tests confirmed reduced drag when boats moved with their ratchet orientation, with ~10% lower drag coefficients compared to smooth boats.
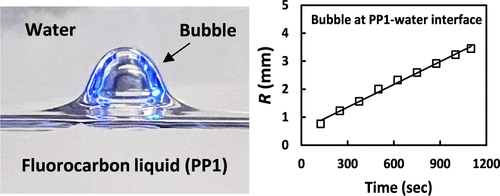
Spontaneous bubble growth inside high-saturation-vapor-pressure and high-air-solubility liquids and emulsion droplets
Spontaneous bubble growths in liquids are usually triggered by rapid changes in pressure or temperature that can lead to liquid gas supersaturation. Here, we report alternative scenarios of the spontaneous growths of bubbles inside a high-saturation-vapor-pressure and high-air-solubility perfluorocarbon liquid (PP1) that were observed under ambient quiescent conditions. First, we investigate spontaneous bubble growth inside the single PP1 phase, which was left to evaporate freely. The bubble growth is explained by the difference in the PP1 vapor pressure inside the bubble and that above the freely evaporating PP1 interface. Next, we study the bubble growth inside the liquid PP1 covered with a layer of a second air-saturated immiscible liquid: low-air-solubility water or higher-air-solubility ethanol. In both cases, the bubble growth rates were accelerated, indicating mass transfer of air from the water or ethanol phases to the PP1 phase. The bubble growth rates significantly increase for bubbles trapped at the PP1–water or PP1–ethanol interfaces due to faster air diffusion through the thin PP1 liquid films separating the bubbles from the upper phases. Finally, we consider the case of bubbles inside millimeter-sized PP1 emulsion droplets in water or ethanol. The bubble growth inside the droplet leads to an increase in the PP1 droplet’s effective buoyancy and to the detachment of the droplets from the substrate. The observed bubble growth rate in the case of emulsion droplets was much faster for PP1 droplets in ethanol than for PP1 droplets in water (minutes vs hours). The underlying physical mechanism of the increase of bubble volumes is the spontaneous mass transfer of both air and PP1 vapor to the bubbles produced by a colloidal diffusion pump effect.
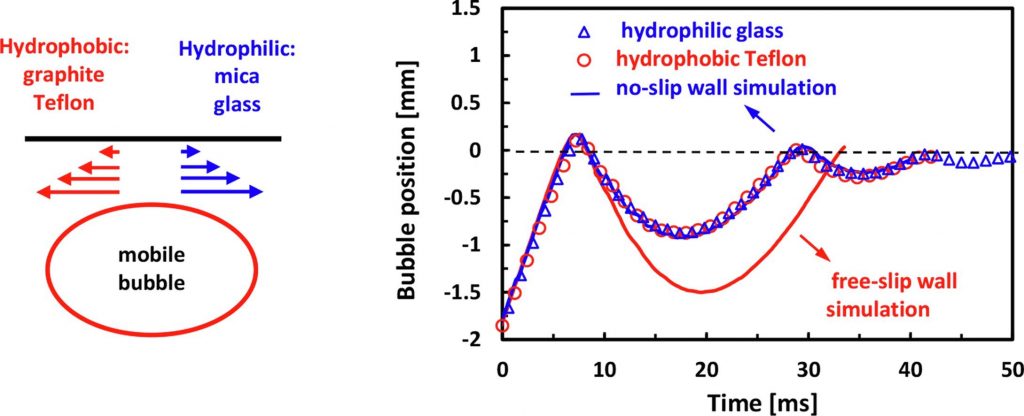
Bouncing bubbles do not show water slip on smooth hydrophobic surfaces
Hypothesis: The presence of hydrodynamic slip of water on smooth hydrophobic surfaces has been debated
intensely over the last decades. In recent experiments, the stronger bounce of free-rising bubbles from smooth
hydrophobic surfaces compared to smooth hydrophilic surfaces was interpreted as evidence for a significant
water slip on smooth hydrophobic surfaces.
Experiments: To examine the possible water-slip effect, we conduct well-controlled experiments comparing the
bouncing dynamics of millimeter-sized free-rising bubbles from smooth hydrophobic and hydrophilic surfaces.
The hydrophobic surfaces were graphite or Teflon, and the hydrophilic surfaces were glass or mica. To avoid
contamination, the experiments were conducted in pure water without any surface-active additives. Numerical
simulations were also used to compare the bounce of the bubble from a no-slip and free-slip walls.
Finding: Our experiments show that the free-rising bubbles in pure water bounce identically from the smooth
hydrophobic graphite or Teflon surfaces as from smooth hydrophilic mica or glass surfaces. The bubble bounce
from all four surfaces is in excellent agreement with the numerical simulation of a bubble bouncing from a flat,
no-slip wall. At the same time, numerical simulations for bubbles bouncing from a free-slip wall predict up to
two-fold stronger bouncing amplitudes. Our experiments and numerical simulations, including estimates of the
shear rates, confirm the no-slip boundary condition for water on smooth hydrophobic surfaces.
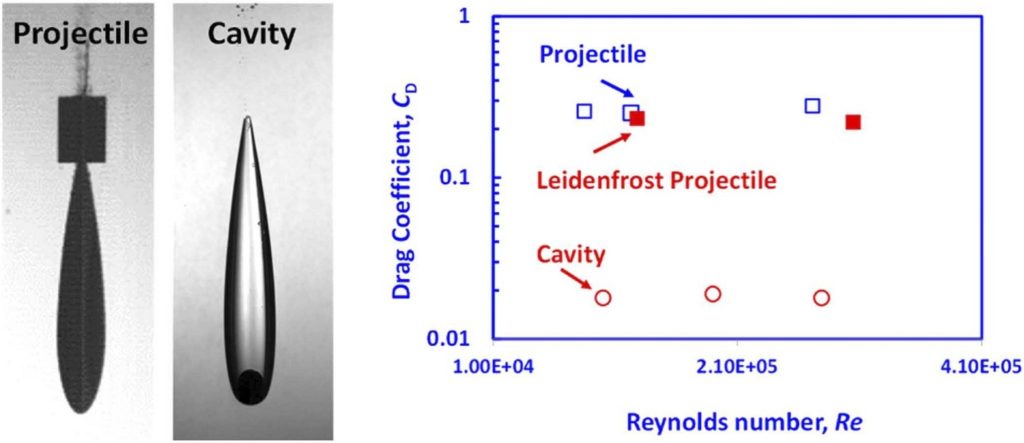
Leidenfrost spheres, projectiles, and model boats: Assessing the drag reduction by superhydrophobic surfaces
Superhydrophobic surfaces are expected to reduce drag on bluff bodies moving in water due to the introduction
of a thin air layer around the solid and the resulting partial slip boundary condition. The use of Leidenfrost vapor
layers, sustained on the surface of a heated metal body is a reliable method to estimate the maximum drag
reduction possible due to such air layers. In the past such an approach was used to estimate the drag reduction on
a free-falling heated sphere, in which case the form drag is the lead component of the drag force. Here, we extend
this approach to evaluate the effect of the thin gas layers on the hydrodynamic drag of free-falling streamlined
projectiles and towed model boats, where the form drag is minimal, and the skin friction drag is the lead
component of the drag force. By comparing the drag for streamlined bodies with and without sustained airlayers, we see only incremental drag reductions, for the sub-critical Reynolds number tested herein. The same
is true for towed model boats. These results hold both for superhydrophobic surface treatments and Leidenfrost
vapor layers. Thus, we concluded that for the investigated range of sub-critical Reynolds numbers, the skin
friction drag is less sensitive to the effect of the thin gas layers compared to the form drag. These novel findings
have important implications for the practical potential of energy savings using gas layers sustained on super
hydrophobic surfaces.
Why bubbles coalesce faster than droplets: The effects of interface mobility and surface charge
Air bubbles in pure water appear to coalesce much faster compared to oil emulsion droplets at the same water solution conditions. The main factors explaining this difference in coalescence times could be interface mobility and/or pHdependent surface charge at the water interface. To quantify the relative importance of these effects, we use high-speed imaging to monitor the coalescence of free-rising air bubbles with the water− air interface as well as free-falling fluorocarbon-oil emulsion droplets with a water−oil interface. We measure the coalescence times of such bubbles and droplets over a range of different water pH values (3.0, 5.6, 11.0). In the case of bubbles, a very fast coalescence (milliseconds) is observed for the entire pH range in pure water, consistent with the hydrodynamics of fully mobile interfaces. However, when the water−air interface is immobilized by the deposition of a monolayer of arachidic acid, the coalescence is significantly delayed. Furthermore, the coalescence times increase with increasing pH. In the case of fluorocarbon-oil droplets, the coalescence is always much slower (seconds) and consistent with immobile interface coalescence. The fluorocarbon droplet’s coalescence time is also pH-dependent, with a complete stabilization (no coalescence) observed at pH 11. In the high electrolyte concentration, a 0.6 M NaCl water solution, bubbles, and droplets have similar coalescence times, which could be related to the bubble interface immobilization at the late stage of the coalescence process. Numerical simulations are used to evaluate the time scale of mobile and immobile interface film drainage.


