Fatmegyul S. Mustan, Ph.D.
Interests
- Molecular dynamics simulations
- Drug solubilization
- Suface properties
- Rheology and stability of foams and emulsions
Bio
Publications
Most recent publications
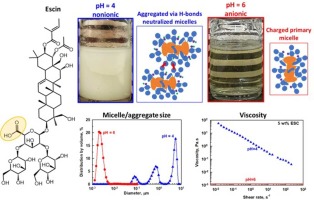
Escin solutions: Effects of pH and electrolytes on their behavior
Escin is a triterpenoid saponin with one carboxyl group which is non-ionized at pH 4.7. The major aim of the current study is to determine how the electrolyte concentration affects the properties of concentrated escin solutions (5 wt% and 10 wt%) at pHs of 4, 6, and 8. Ionized escin molecules at pH >4.7 form charged micelles that repel one another when there is no added electrolyte and solutions remain clear and stable for more than a month. Lowering the pH to 4 leads to formation of uncharged micelles. These micelles attract each other and form inter-micellar hydrogen bonds, which enable formation of micrometer aggregates that cause turbidity and phase separation. The addition of background electrolytes to the solutions at pHs of 6 and 8 screens the electrostatic repulsion between micelles, causing partial aggregation of the micelles and gelation of solutions. As the salt concentration increases, the viscosity of the escin solution also increases, reaching a maximum—similar to the behavior observed with conventional surfactants. However, the mechanism behind this viscosity maximum is different. In solutions of conventional surfactants, the maximum is due to the formation of worm-like micelles, whereas the maximum for escin solutions is due to formation of a network of escin aggregates that imparts yield stress and elasticity to the solution. These dispersions remain stable for at least one month at room temperature and can be used as cosmetic and detergent formulations.
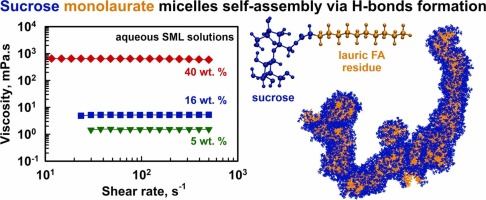
Sucrose monolaurate self-assembly via hydrogen bonding: role of surfactant concentration and urea
Sugar esters, a class of surfactants derived from renewable resources, have attracted significant attention due to their biodegradability, low toxicity, and broad applications in food, cosmetic, and pharmaceutical formulations. Despite their widespread use, the phase behavior of these compounds in aqueous systems remains incompletely understood. In this study, we investigate the self-assembly of a nonionic sucrose ester of lauric acid in 1–40 wt% concentration range using rheological measurements, dynamic light scattering, X-ray scattering, DOSY NMR, and molecular dynamics simulations. Formation of spherical micelles with a diameter of 5.4 nm is observed at low surfactant concentrations, driven by hydrophobic interactions between the alkyl tails. These solutions exhibit Newtonian flow behavior with viscosities close to that of pure water. However, the viscosity increases from 5 mPa.s at 16 wt% to 640 mPa.s at 40 wt%, while the Newtonian character persists even at 40 wt%. This behavior is explained with the formation of interconnected, thread-like micellar structures of (almost) spherical micelles that largely preserve their distinctiveness, resembling the “pearl necklace” arrangement known for polymer systems. The main driving force for this supramolecular organization was found to be the hydrogen bonding between sucrose headgroups. The addition of 6 M urea, a known hydrogen bond disruptor, significantly reduces micelle clustering and the viscosity decreases to 150 mPa.s at 40 wt% concentration, supporting the proposed aggregation mechanism. These findings contribute to a deeper understanding of the self-assembly behavior of sucrose esters in aqueous environment and highlight their potential for controlled aggregation in practical formulations.
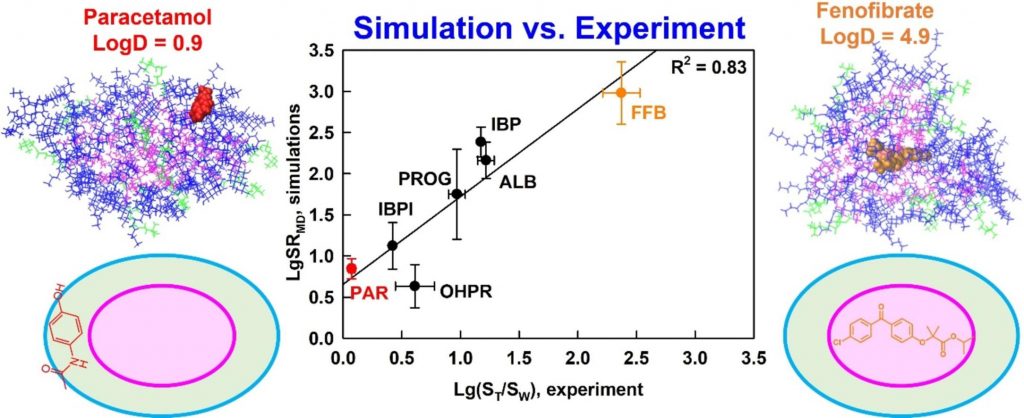
Understanding drug solubilization in intestinal mixed micelles through molecular dynamics simulations
Hypothesis
Solubilization is a fundamental process that underpins various technologies in the pharmaceutical and chemical industry. However, knowledge of the location, orientation and interactions of solubilized molecules in the micelles is still limited. We expect all-atom molecular dynamics simulations to improve the molecular-level understanding of solubilization and to enable its in silico prediction.
Methods
The solubilization of six drugs in intestinal mixed micelles composed of taurocholate and dioleoyl phosphatidylcholine was simulated by molecular dynamics in explicit water and measured experimentally by liquid chromatography. The location and orientation of the solubilized drugs were visualized by cumulative radial distribution functions and interactions were characterized by radial distribution function ratios and hydrogen bonding.
Findings
A new simulation-derived parameter was defined, which accounts for drug-micelle and drug-water interactions and correlates (R2 = 0.83) with the experimentally measured solubilization. Lipophilicity was found to govern the location of all drugs in the micelle (hydrophobic core, palisade layer or on the surface), while hydrogen bonding was crucial for orientation and solubilization of two of the molecules. The study demonstrates that explicit, hydrogen bond-forming water molecules are vital for accurate prediction of solubilization and provides a comprehensive framework for quantitative studies of drug location and orientation within the micelles.
Taurodeoxycholate aggregation explored by molecular dynamics: Primary-to-secondary micelle transition and formation of mixed micelles with fatty acids
Micelles formed by bile salts in aqueous solution are important for the solubilization of hydrophobic molecules in the gastrointestinal tract. The molecular level information about the mechanism and driving forces for primary-to-secondary micelle transition is still missing. In the current study, the micelle formation of 50 mM solutions of taurodeoxycholate (TDC) is studied by atomistic molecular dynamics simulations. It is shown that primary micelles with an aggregation number of 8-10 emerge and persist within the first 50 ns. Then, they coalesce to form secondary micelles with an aggregation number of 19 molecules. This transition is governed by hydrophobic interactions, which significantly decrease the solvent-accessible surface area per molecule in the secondary micelles. The addition of monomers of the sodium salt of fatty acids (FAs), as agents aiding hydrophobic drug delivery, to secondary TDC micelles results in the co-existence of mixed FA-TDC and pure FA micelles. The studied saturated FAs, with chain lengths of C14:0 and C18:0, are incorporated into the micelle core, whereas TDC molecules position themselves around the FAs, forming a shell on the micelle surface. In contrast, the tails of the C18:1 unsaturated fatty acid mix homogeneously with TDC molecules throughout the entire micelle volume. The latter creates a very suitable medium for hosting hydrophobic molecules in the micelles containing unsaturated fatty acids.
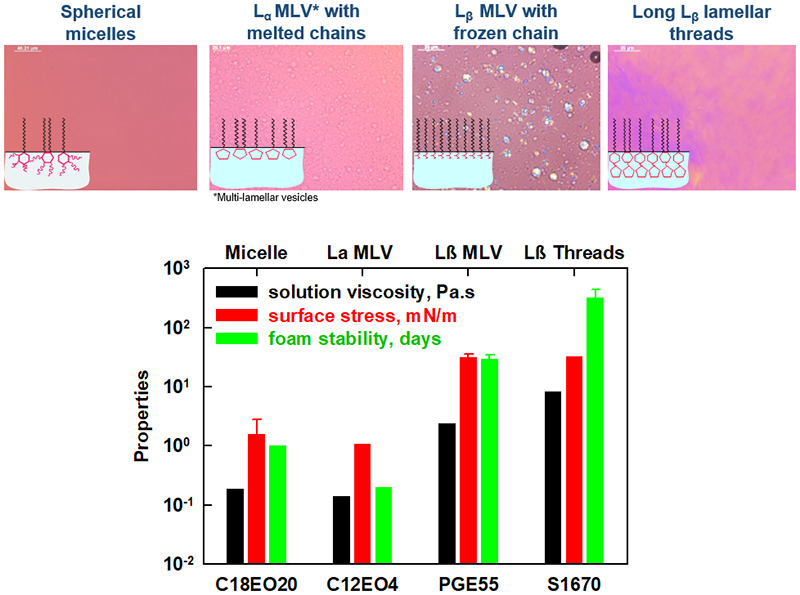
Interplay between bulk aggregates, surface properties and foam stability of nonionic surfactants
In our previous study (Mustan et al. 2021) we showed that foams formed from two oil-soluble nonionic surfactants (Span 60 and Brij 72) can remain stable for more than 10 days at room temperature at high sugar concentration. The major aim of the current study is to reveal the interrelation between the surfactant structure and foam stability by investigating 6 polyoxyethelene alkyl ethers and 12 fatty acid esters with a wide variety of hydrophobic chain lengths (C12; C16; C18 and C18:1) and hydrophilic head-groups (sorbitol, glycerol, sucrose). Foams stable for more than 100 days at room temperature are obtained when sucrose palmitate or stearate (P1670 or S1670) are used as surfactants. This exceptional foam stability is related to the gelation of the aqueous phase and to the formation of solid adsorption layer with zero surface tension upon compression, thus preventing water drainage and decelerating the bubble Ostwald ripening. The foam stability decreases with (i) increasing the number of EO groups in polyoxyethylene alkyl ethers and in fatty acid sorbitan esters; (ii) decreasing the number of C-atoms in the surfactant tail for all studied surfactants; (iii) addition of double bond in the surfactant tail. The lower foam stability in all three cases is related to the worse packing of the surfactant molecules within the adsorption layer, leading to faster Ostwald ripening and subsequent bubble coalescence. The diesters present as admixture in the fatty acid esters play an important role in the foam stabilization by further compacting the adsorption layers and lowering the rate of Ostwald ripening. These conclusions can be used as a predictive tool for surfactant selection in the development of food or pharmaceutical foam concentrates that can be diluted before final use.