
Dora T. Dimitrova, Chemist
Interests
- Equilibrium Surface Tension
- Methods for Dynamic Surface Tension Measurement
Publications
Most recent publications
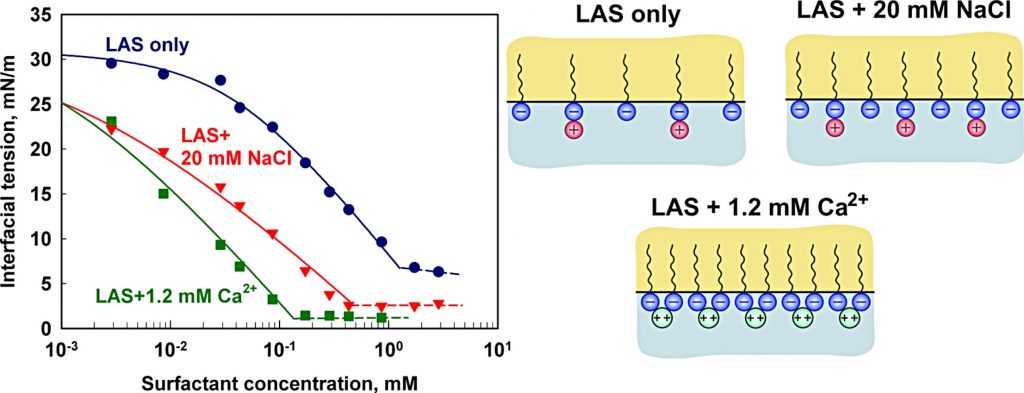
Adsorption of linear alkyl benzene sulfonates on oil-water interface: Effects of Na+, Mg2+ and Ca2+ ions
Linear alkyl benzene sulfonates (LAS) are among the most important industrial and house-hold surfactants. Here we study the LAS adsorption properties at oil-water interface in the presence of divalent counter-ions (Mg2+ and Ca2+). Interfacial tension data are obtained and interpreted using a detailed thermodynamic model for surfactant adsorption, which explicitly accounts for counter-ion binding (Kralchevsky et al. Langmuir 15 (1999) 2351). The obtained results show that the hardness ions (i) reduce very significantly the area-per-molecule in the adsorption layer; (ii) reduce strongly the magnitude of the negative surface potential, neutralizing almost completely the adsorbed LAS molecules; (iii) bind strongly to the adsorption layer via both electrostatic attraction and specific attraction of magnitude around 3.5kT. The limiting area-per-molecule at oil-water interface is shown to be significantly larger than the respective area at air-water interface. The latter result indicates that the oil molecules are able to intercalate in between the surfactant molecules in the adsorption layer and, thus, to disrupt the molecular packing at the oil-water interface.
Equations of state and adsorption isotherms of low molecular non-ionic surfactants
After a brief analysis of the three most widely used equations of state and adsorption isotherms, (those of van der Waals, Frumkin and Helfand-Frisch-Lebowitz with second virial coefficient) we analyze the definitions and the values of the main adsorption parameters (adsorption constant Ks, minimum area per molecule α and interaction constant β) and derive new expressions for some of them. Since it turned out that all three adsorption isotherms perform rather poorly, when used to interpret adsorption data, we applied also three more general equations-one was derived for localized adsorption long ago, but we had to derive two new equations for non-localized adsorption. We subject all 6 equations-the simple ones and the three generalized, to rigorous numerical analysis and discussion of the results. Our overall conclusion is that in some cases all equations can describe qualitatively the observed phenomena, but only the new equation of state, proposed by us for non-localized adsorption on fluid interface (and the respective adsorption isotherm), lead to correct quantitative description of the adsorption and the related parameters. In Appendix A we analyze the shortcomings and the source of errors in the fitting procedures.
Method and device for speedily forming fluid interface, and use of this device for determining nature of interface between liquid to liquid, and gas to liquid
Role of the counterions on the adsorption of ionic surfactants
A theory accounting for the effect of the counterions on the adsorption constant, K, is proposed. The experimental values of K were determined by using surface and interfacial tension isotherms measured by us or available in the literature. By accounting for the adsorption energy u0 of the counterion, a generalization of Gouy equation and a modified expression for the adsorption constant, K, are derived. The adsorption energy is calculated from the London equation, which involves the polarizabilities α0i and the ionization potentials Ii of the respective components and the radius Rh of the hydrated ion. By careful analysis of the available experimental data for α0i, Ii and Rh, coupled with some reasonable hypothesis, we succeeded to obtain linear dependences between the calculated values of u0 and the experimental data for lnK with slopes rather close to the theoretical ones. The obtained results for u0 were used to calculate the disjoining pressure isotherms of foam films stabilized by DTAF, DTACl and DTABr. It turned out that the type of the counterion has significant effect on the disjoining pressure.
Ionic surfactants on fluid interfaces: Determination of the adsorption; Role of the salt and the type of the hydrophobic phase
In this work we describe a simple thermodynamic method for determination of the adsorption (amount per unit area) of ionic surfactant. The latter is obtained from the interfacial tension isotherm measured in the presence of arbitrary (and fixed) concentration of inorganic electrolyte. Polynomial fit of the interfacial tension versus the logarithm of the mean ionic activity is combined with the Gibbs adsorption equation written in a form suitable for arbitrary content of salt. This procedure is an extension of the approach of Rehfeld [J. Phys. Chem. 71 (1967) 738]. We have performed measurements with sodium dodecyl sulfate (SDS) on water/air and water/oil (n-hexadecane and Soybean Oil) boundaries, at different salt concentrations (10 and 150 mM NaCl). Wilhelmy plate method was used for the water/air measurements; for water/oil we applied drop shape analysis with pendant drops. The obtained isotherms, together with literature data, are processed and the adsorption is determined. The results are compared and discussed in view of the role of the salt and the type of the hydrophobic phase. On oil/water boundaries the adsorption is always lower than that on air/water surface; addition of inert electrolyte increases the adsorption. We analyze theoretically the asymptotic behavior of the adsorption as a function of the solute concentrations in the limit of high surface coverage. The treatment is based on models existing in the literature (Langmuir isotherm with account for the counterion binding, as formulated by Kalinin and Radke [Colloids Surf. A 114 (1996) 337]; the activity coefficients were taken into consideration in the frames of the Debye-Hückel theory). The obtained asymptotic functional dependence of the adsorption is used for fitting of data. The agreement is always good, in the concentration region below and near the critical micellization concentration (CMC). From the fits we determine the limiting adsorption at maximum coverage (i.e., at saturation); therefrom, the degree of coverage of the interface with surfactant is estimated. It turns out that at the CMC the coverage is lower than about 90%. Thus, we confirm literature results for absence of saturation with ionic surfactants at the CMC. The dependence of the surface coverage upon the mean ionic activity is rather insensitive toward the type of the fluid interface (air/water, oil/water with different hydrocarbons), and the salt concentration.

