Volatile aroma surfactants: The evaluation of the adsorption-evaporation behavior under dynamic and equilibrium conditions
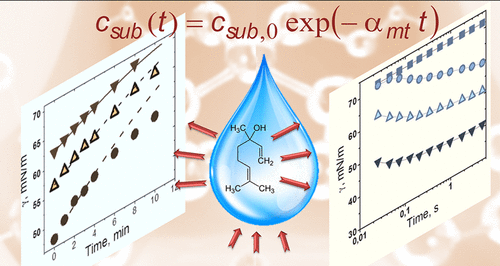
Multicomponent heterogeneous systems containing volatile amphiphiles are relevant to the fields ranging from drug delivery to atmospheric science. Research presented here discloses the individual interfacial activity and adsorption-evaporation behavior of amphiphilic aroma molecules at the liquid-vapor interface. The surface tension of solutions of nonmicellar volatile surfactants linalool and benzyl acetate, fragrances as such, was compared with that of the conventional surfactant sodium dodecyl sulfate (SDS) under equilibrium as well as under no instantaneous equilibrium, including a fast-adsorbing regime. In open systems, the increase in the surface tension on a time scale of ∼10 min is evaluated using a phenomenological model. The derived characteristic mass transfer constant is shown to be specific to both the desorption mechanism and the chemistry of the volatile amphiphile. Fast-adsorbing behavior disclosed here, as well as the synergetic effect in the mixtures with conventional micellar surfactants, justifies the advantages of volatile amphiphiles as cosurfactants in dynamic interfacial processes. The demonstrated approach to derive specific material parameters of fragrance molecules can be used for an application-targeted selection of volatile cosurfactants, e.g., in emulsification and foaming, inkjet printing, microfluidics, spraying, and coating technologies.

