Temperature response of sucrose palmitate solutions: Role of ratio between monoesters and diesters
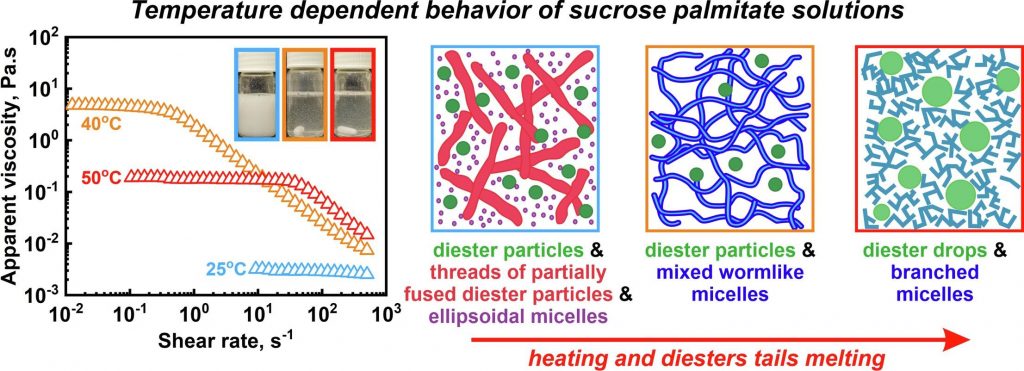
Hypothesis: Aqueous solutions of long-chain water-soluble sucrose ester surfactants exhibit non-trivial response to temperature variations, revealing a peak in viscosity around 40–50 ◦C. While previous investigations have explored the structures within sucrose stearate systems at various constant temperatures, a comprehensive understanding of the entire temperature dependence and the underlying molecular factors, contributing to this phenomenon is currently missing. Experiments: Temperature dependent properties and supramolecular structures formed in aqueous solutions of commercial sucrose palmitate were examined using SAXS/WAXS, DSC, optical microscopy, rheological measurements, NMR, and cryo-TEM. Findings: The underlying mechanism governing this unusual behavior is revealed and is shown to relate to the mono- to di-esters ratio in the solutions. Solutions primarily containing sucrose monoesters (monoesters molecules ≳ 98% of all surfactant molecules) exhibit behavior typical of nonionic surfactants, with minimal changes with temperature. In contrast, the coexistence of mono- and di-esters results in the formation of discrete monodisperse diester particles and a network of partially fused diester particles at low temperature. As the temperature approaches the diesters’ melting point, wormlike mixed micelles form, causing a viscosity peak. The height of this peak increases significantly with the diester concentration. Further temperature increase leads to fluidization of surfactant tails and formation of branched micelles, while excess diester molecules phase separate into distinct droplets.

