Sucrose monolaurate self-assembly via hydrogen bonding: role of surfactant concentration and urea
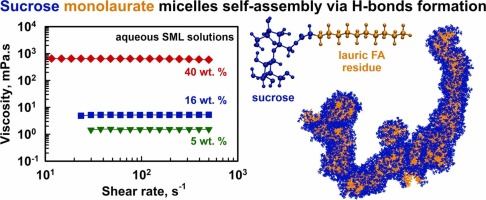
Sugar esters, a class of surfactants derived from renewable resources, have attracted significant attention due to their biodegradability, low toxicity, and broad applications in food, cosmetic, and pharmaceutical formulations. Despite their widespread use, the phase behavior of these compounds in aqueous systems remains incompletely understood. In this study, we investigate the self-assembly of a nonionic sucrose ester of lauric acid in 1–40 wt% concentration range using rheological measurements, dynamic light scattering, X-ray scattering, DOSY NMR, and molecular dynamics simulations. Formation of spherical micelles with a diameter of 5.4 nm is observed at low surfactant concentrations, driven by hydrophobic interactions between the alkyl tails. These solutions exhibit Newtonian flow behavior with viscosities close to that of pure water. However, the viscosity increases from 5 mPa.s at 16 wt% to 640 mPa.s at 40 wt%, while the Newtonian character persists even at 40 wt%. This behavior is explained with the formation of interconnected, thread-like micellar structures of (almost) spherical micelles that largely preserve their distinctiveness, resembling the “pearl necklace” arrangement known for polymer systems. The main driving force for this supramolecular organization was found to be the hydrogen bonding between sucrose headgroups. The addition of 6 M urea, a known hydrogen bond disruptor, significantly reduces micelle clustering and the viscosity decreases to 150 mPa.s at 40 wt% concentration, supporting the proposed aggregation mechanism. These findings contribute to a deeper understanding of the self-assembly behavior of sucrose esters in aqueous environment and highlight their potential for controlled aggregation in practical formulations.

