Solubility limits and phase diagrams for fatty alcohols in anionic (SLES) and zwitterionic (CAPB) micellar surfactant solutions
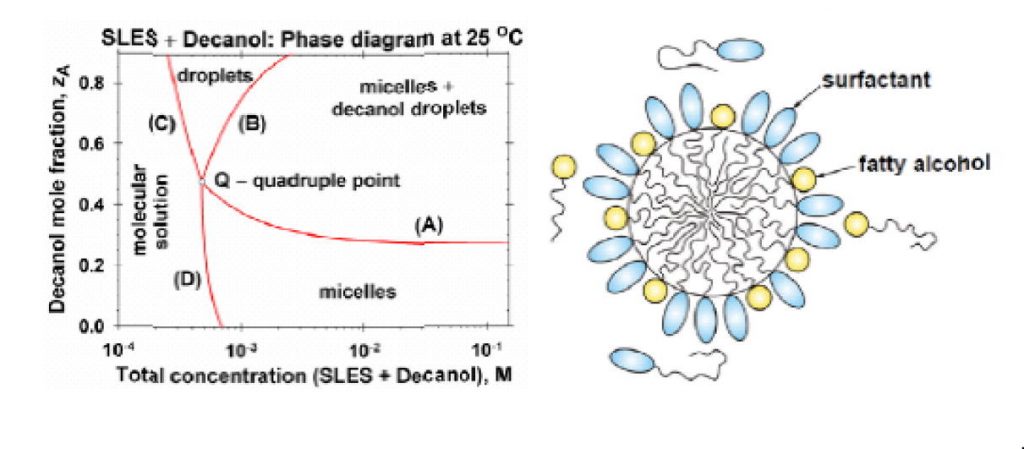
By analysis of experimental data, a quantitative theoretical interpretation of the solubility limit of medium- and long-chain fatty alcohols in micellar solutions of water-soluble surfactants is presented. A general picture of the phase behavior of the investigated systems is given in the form of phase diagrams. The limited solubility of the fatty alcohols in the micelles of conventional surfactants is explained with the precipitation of their monomers in the bulk, rather than with micelle phase separation. The long chain fatty alcohols (with n= 14, 16 and 18 carbon atoms) exhibit an ideal mixing in the micelles of the anionic surfactant sodium laurylethersulfate (SLES) and the zwitterionic surfactant cocamidopropyl betaine (CAPB) at temperatures of 25, 30, 35 and 40. °C. Deviations from ideality are observed for the alcohols of shorter chain (n= 10 and 12), which can be explained by a mismatch with the longer chains of the surfactant molecules. Using the determined thermodynamic parameters of the systems, their phase diagrams are constructed. Such a diagram consists of four domains, viz. mixed micelles; coexistent micelles and precipitate (dispersed crystallites or droplets); precipitate without micelles, and molecular solution. The four boundary lines intersect in a quadruple point, Q. For ionic surfactants (like SLES), a detailed theory for calculating the boundary lines of the phase diagrams is developed and verified against data for the positions of the kinks in surface tension isotherms. The theory takes into account the electrostatic interactions in the micellar solutions and the effect of counterion binding. The results can be useful for a quantitative interpretation and prediction of the phase behavior of mixed solutions of two (or more) surfactants, one of them being water soluble and forming micelles, whereas the other one has a limited water solubility, but readily forms mixed micelles with the former surfactant.

