Role of lysophospholipids on the interfacial and liquid film properties of enzymatically modified egg yolk solutions
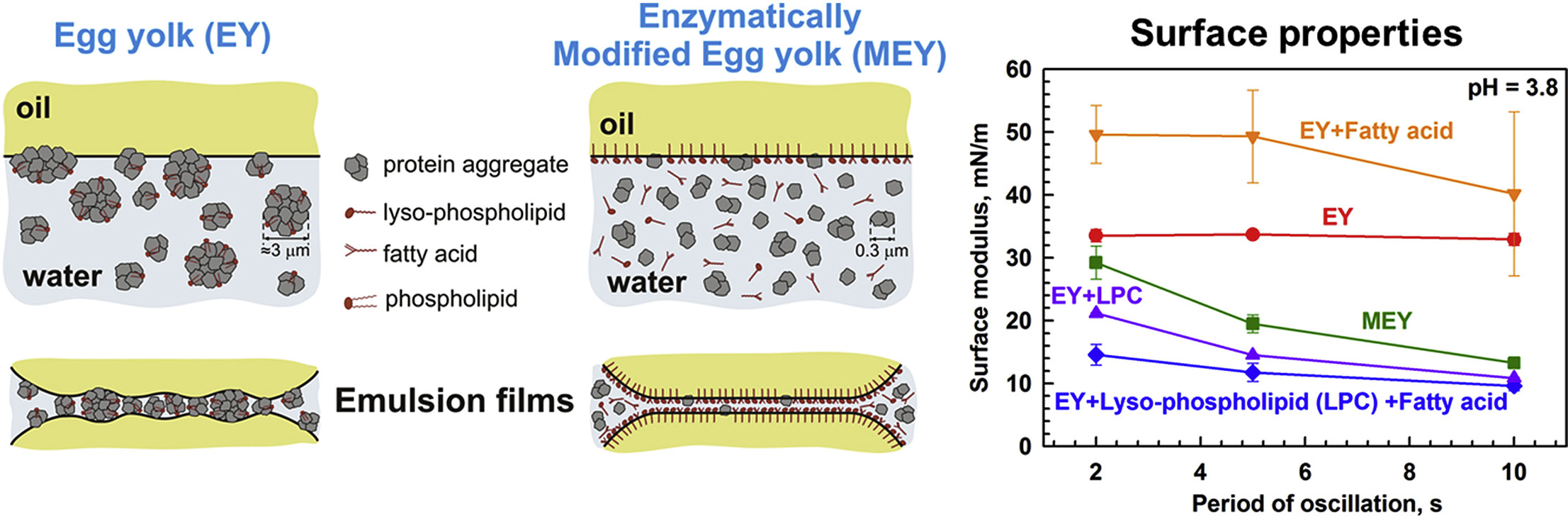
This study aims to clarify and explain the similarities and differences in the behavior of adsorption layers of native egg yolk (EY) and enzymatically modified egg yolk (MEY) at a soybean oil-water interface. For this purpose, the interfacial tension and the surface dilatational modulus of EY and MEY solutions are measured and compared. The interactions between two adsorption layers, formed from these solutions on an oil-water or air-water interface, are also studied by optical observations of thin foam and emulsion films, formed in a capillary cell. The chemical composition, the electrophoretic mobility of the molecular aggregates, and the rheological properties of the egg yolk solutions are also characterized. Adsorption layers formed from MEY solutions display a faster rate of adsorption, lower dilatational surface moduli and higher equilibrium surface tension. The enzymatic modification of egg yolk also leads to formation of much thinner foam and emulsion films and to faster film thinning. The observed differences between EY and MEY are explained by assuming that the interfacial properties of MEY are governed mostly by the lysophospholipids and oleic acid, which appear as reaction products of the enzymatic modification of EY. The latter assumption is unambiguously proven by chemical analysis of the MEY solutions and by deliberate addition of lysophospholipids and oleic acid to the non-modified EY solutions. Even at relatively low concentrations, the lysophospholipids and oleic acid change the interfacial and film properties of the EY solutions, making them very similar to those of the enzymatically modified egg yolk.

