Role of dispersion nanostructure for bubble dissolution under pressure
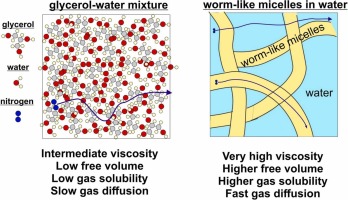
Understanding the factors that affect bubble dissolution under pressure is crucial for the pneumatic transport of dispersions. This study probes the kinetics of air dissolution, the air solubility at a given pressure, and the gas diffusion due to bubble dissolution to elucidate the molecular mechanisms of gas transport in liquid dispersions with varied structures and viscosities. We achieve our aims by using water-glycerol mixtures, silicone oils with different viscosities, surfactant solutions containing worm-like micelles and having different macroscopic viscosities, particle suspensions, and surfactant solutions capable of forming a condensed adsorption layer on the bubble surfaces at ambient conditions. The results show that the dissolution rate does not depend on the macroscopic viscosity for silicone oils and solutions containing worm-like micelles, indicating that gas diffusion occurs faster than the movement of big polymeric molecules and worm-like micelles. We could predict the experimentally determined diffusion coefficients by accounting for free volume in these media and using the equation for Knudsen diffusion. We show that one way to decrease the rate of bubble dissolution under pressure is to add surfactants, which can decrease the permeability of the adsorption layer formed on the bubble surface by forming a condensed adsorption layer.

