Quantitative characterization of the mass transfer of volatile amphiphiles between vapor and aqueous phases: Experiment vs theory
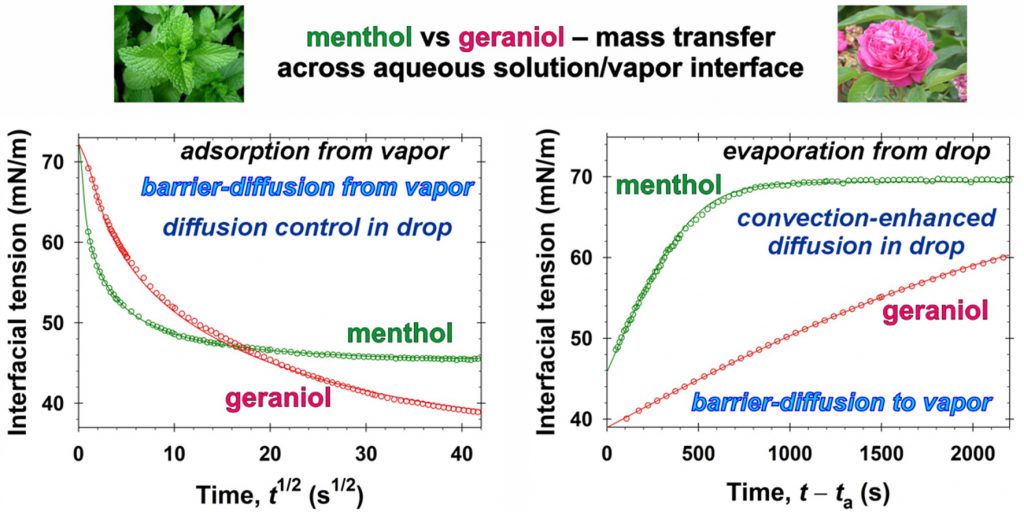
The class of volatiles, which possess low saturated vapor pressures, appreciable solubilities in water, and well pronounced surface activities, have gained wide applications in diverse areas of industry, cosmetics, and medicine. One way to qualitatively characterize their mass transfer between vapor and aqueous solutions is to measure the relaxation of the interfacial tension, σ, with time, t, under different nonequilibrium initial conditions. This approach is applied in the present work for geraniol and menthol. By means of combining σ(t) data with the respective equilibrium surface tension isotherms, the instantaneous values of the fragrance adsorption, Γ(t), have been determined. Quantitative characterization of the geraniol and menthol mass transfers in the case of adsorption from vapor to aqueous drops is achieved by using a mixed barrier-diffusion model. The obtained values of the rates of adsorption and desorption are compared with those reported in the literature for benzyl acetate, linalool, and citronellol. In the case of evaporation of the volatiles from their saturated aqueous solutions to the ambient atmosphere, the mass transfer is found to be driven both by mixed barrier-diffusion and by convection-enhanced mechanisms – depending on the air humidity. The quantitative description of the evaporation of volatile molecules is modelled theoretically by adsorption rate constants. In order to achieve the reported model representations, complex numerical calculations are implemented. On the other hand, having in mind the cases when one wishes to avoid extensive computational work, we developed a simple semiempirical model suitable for all five studied fragrances. This simplified approach is convenient for the express comparison and characterization of the evaporation rates. The obtained physicochemical parameters related to the evaporation and condensation of volatiles are important for the rigorous modeling of their complex mixed solutions of practical interest. The semiempirical model could be used for the quantitative classification of volatile molecules with respect to their ability to evaporate.

