Interplay between cosurfactants and electrolytes for worm-like micelles formation
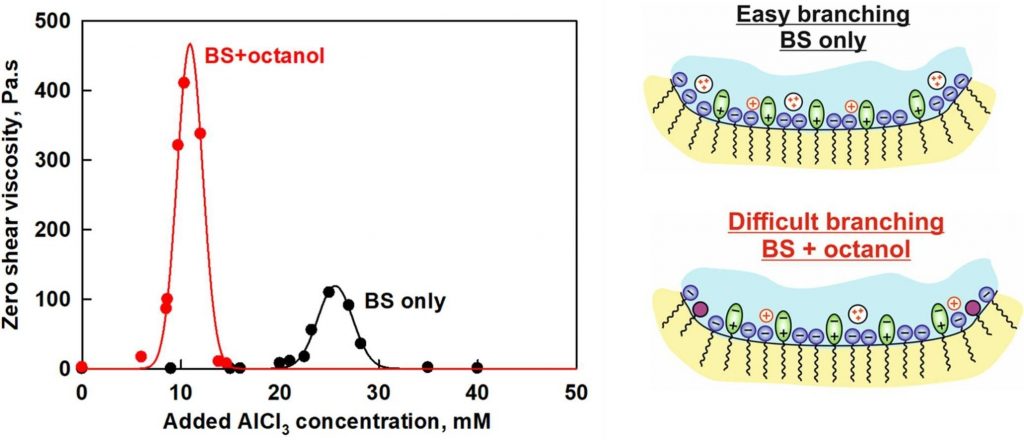
The rheological response of surfactant solutions containing a mixture of anionic and zwitterionic surfactants, in the presence of shorter-chain cationic and nonionic co-surfactants and various counterions was studied experimentally and described theoretically by developing the model that accounts for the competitive adsorption of different monovalent and divalent counterions, as well as the inclusion of co-surfactants within the micelles. This model was used to predict the salt curve dependence of systems with various salt and co-surfactant concentrations and was tested against the experimentally measured salt curves. A good agreement was found between the experimental data and the proposed theoretical model. It was demonstrated that the adsorption energies of counterions on the micellar surfaces remain unchanged with the addition of co-surfactants. However, the conditions for micelle branching are significantly affected, particularly in the presence of divalent and trivalent counterions. The presence of co-surfactants reduces the number of adsorbed divalent ions, thereby diminishing their effect on micelle branching.

