Foamability of aqueous solutions: Role of surfactant type and concentration
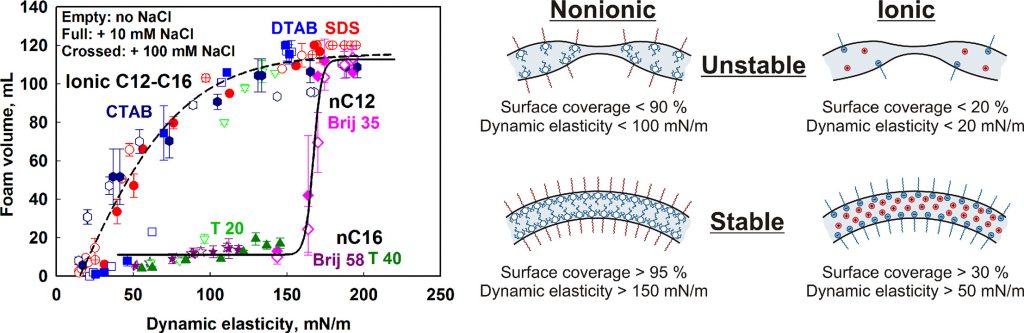
In this paper we study the main surface characteristics which control the foamability of solutions of various surfactants. Systematic series of experiments with anionic, cationic and nonionic surfactants with different head groups and chain lengths are performed in a wide concentration range, from 0.001 mM to 100 mM. The electrolyte (NaCl) concentration is also varied from 0 up to 100 mM. For all surfactants studied, three regions in the dependence of the foamability, VA, on the logarithm of surfactant concentration, lgCS, are observed. In Region 1, VA is very low and depends weakly on CS. In Region 2, VA increases steeply with CS. In Region 3, VA reaches a plateau. To analyse these results, the dynamic and equilibrium surface tensions of the foamed solutions are measured. A key new element in our interpretation of the foaming data is that we use the surface tension measurements to determine the dependence of the main surface properties (surfactant adsorption, surface coverage and surface elasticity) on the surface age of the bubbles. In this way we interpret the results from the foaming tests by considering the properties of the dynamic adsorption layers, formed during foaming. The performed analysis reveals a large qualitative difference between the nonionic and ionic surfactants with respect to their foaming profiles. The data for the nonionic and ionic surfactants merge around two master curves when plotted as a function of the surface coverage, the surface mobility factor, or the Gibbs elasticity of the dynamic adsorption layers. This difference between the ionic and nonionic surfactants is explained with the important contribution of the electrostatic repulsion between the foam film surfaces for the ionic surfactants which stabilizes the dynamic foam films even at moderate surface coverage and at relatively high ionic strength (up to 100 mM). In contrast, the films formed from solutions of nonionic surfactants are stabilized via steric repulsion which becomes sufficiently high to prevent bubble coalescence only at rather high surface coverage (> 90%) which corresponds to related high Gibbs elasticity (> 150 mN/m) and low surface mobility of the dynamic adsorption layers. Mechanistic explanations of all observed trends are provided and some important similarities and differences with the process of emulsification are outlined.

