Encapsulation of fragrances and oils by core-shell structures from silica nanoparticles, surfactant and polymer: Effect of particle size
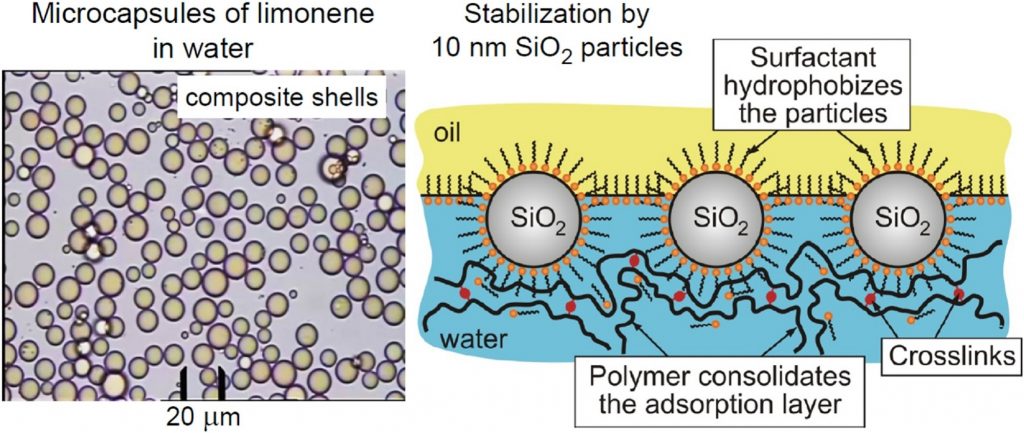
Oils and fragrances can be encapsulated by using composite shells of silica nanoparticles, polymer and surfactant (potassium oleate). The template for the creation of the core-shell structure is a particle stabilized (Pickering) emulsion. The surfactant adsorbs on the nanoparticles and leads to their reversible hydrophobization and adsorption on the oil/water interface. The outer layer of the self-assembled shell represents a layer from crosslinked polymer. The procedure of encapsulation is simple and includes single homogenization by ultrasound of the formulation that contains all ingredients together. The produced capsules have mean radius in the range between 2 and 11 microns. By order of magnitude and trend, the capsule size follows the law of limited coalescence with respect to the dependence on nanoparticle size and concentration. The composite structure of the shells leads also to dependence on the concentrations of added polymer and surfactant. The produced microcapsules are stable when rinsed with pure water of pH in the range 3 – 10. However, if dispersed in water of pH > 11, the microcapsules are destabilized and release their cargo, i.e., they are pH-responsive. Various fragrances and oils, such as limonene, citronellol, benzyl acetate, and sunflower seed oil were encapsulated. The developed methodology could find applications in any field, in which reversible encapsulation of oily substances is needed.

