Cosurfactants for controlling the surface properties of diluted solutions: Interplay with bulk rheology of concentrated solutions
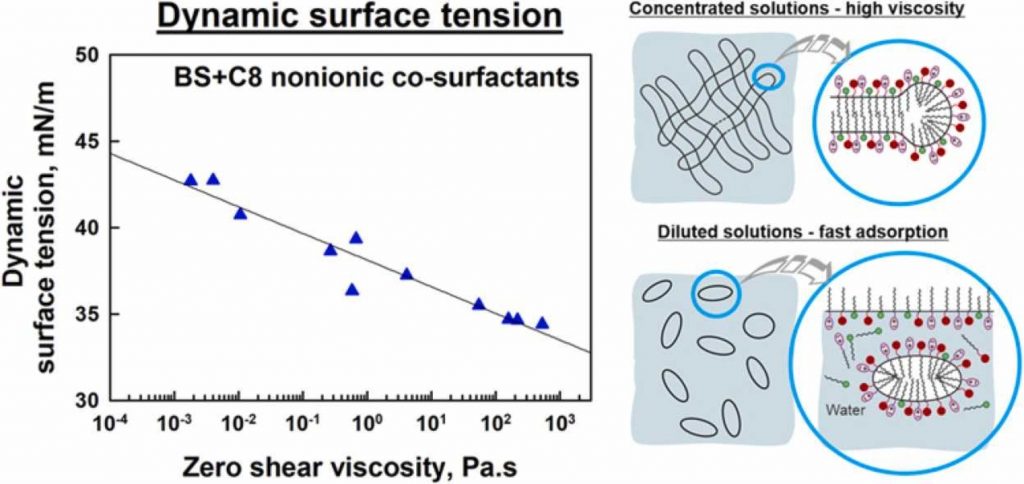
The effect of the fatty alcohols (CnOH), fatty acids (CnAc), alkyltrimethyl ammonium bromides (CnTAB), alkyl sulfates (CnSO4Na) and alkyl acetates (CnAcet), with n varied between 6 and 12 C-atoms, on the surface properties of 0.5 wt% mixed solutions of sodium laurylether sulfate (SLES) and cocoamidopropyl betaine (CAPB) was studied. It was shown that the equilibrium surface tension decreases with increasing the cosurfactant chain-length. For example, the addition of C12OH to SLES+CAPB solution decreases its surface tension from 29 down to 22 mN/m. Both the dynamic surface tension and the characteristic adsorption time pass through a deep minimum when cationic or nonionic cosurfactants with 8 or 10 C-atoms are added. The minimum is particularly deep for nonionic surfactants with small head group (acids and alcohols) which destabilize the micelles and increase the rate of surfactant adsorption. The addition of anionic cosurfactants does not change the dynamic and equilibrium surface tensions of SLES+CAPB system. A strong correlation between the dynamic surface tension of diluted surfactant solutions (0.5 wt%) and the viscosity of the respective concentrated solutions (10 wt%) is established and explained for nonionic cosurfactants.

