Analytical modeling of micelle growth. 5. Molecular thermodynamics of micelles from zwitterionic surfactants
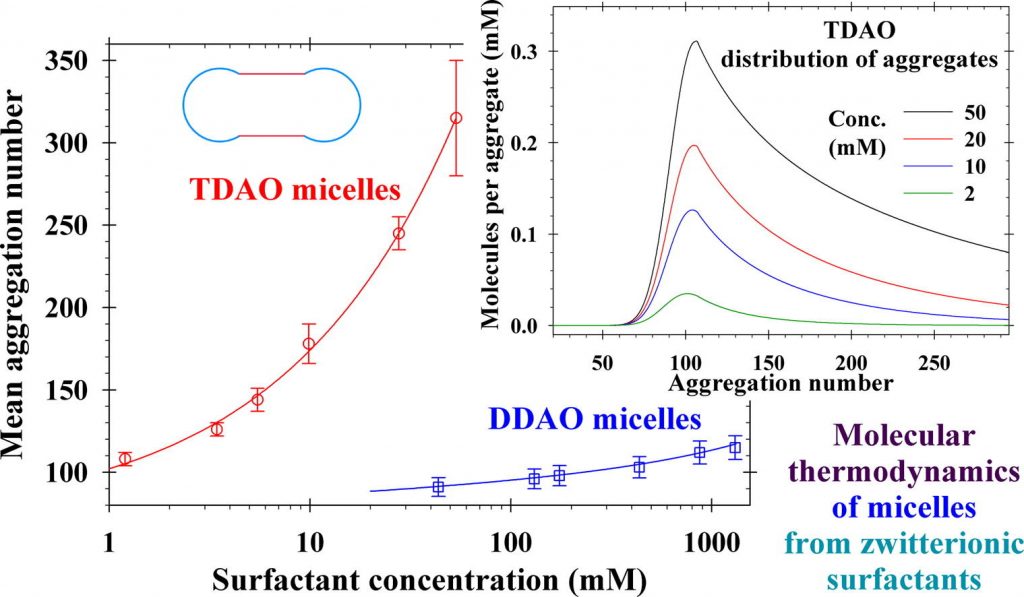
Hypothesis: The critical micelle concentration, aggregation number, shape and length of spherocylindrical micelles in solutions of zwitterionic surfactants can be predicted by knowing the molecular parameters and surfactant concentrations. This can be achieved by upgrading the quantitative molecular thermodynamic model with expressions for the electrostatic interaction energy between the zwitterionic dipoles and micellar hydrophobic cores of spherical and cylindrical shapes. Theory: The correct prediction of the mean micellar aggregation numbers requires precise calculations of the free energy per molecule in the micelles. New analytical expressions for the dipole electrostatic interaction energy are derived based on the exact solutions of the electrostatic problem for a single charge close to a boundary of spherical and cylindrical dielectric media. The obtained general theory is valid for arbitrary ratios between dielectric constants, radii of spheres and cylinders, positions, and orientations of dipoles. Findings: The detailed numerical results show quantitatively the effects of the micelle curvature and dielectric properties of the continuum media on the decrease of the dipole electrostatic interaction energy. Excellent agreement was achieved between the theoretical predictions and experimental data for the critical micelle concentration, size and aggregation number of zwitterionic surfactant micelles. This study can be extended to mixed micelles of zwitterionic and ionic surfactants in the presence of salt to interpret and predict the synergistic effect on the rheology of solutions.

