Analytical modeling of micelle growth. 4. Molecular thermodynamics of wormlike micelles from ionic surfactants: Theory vs. experiment
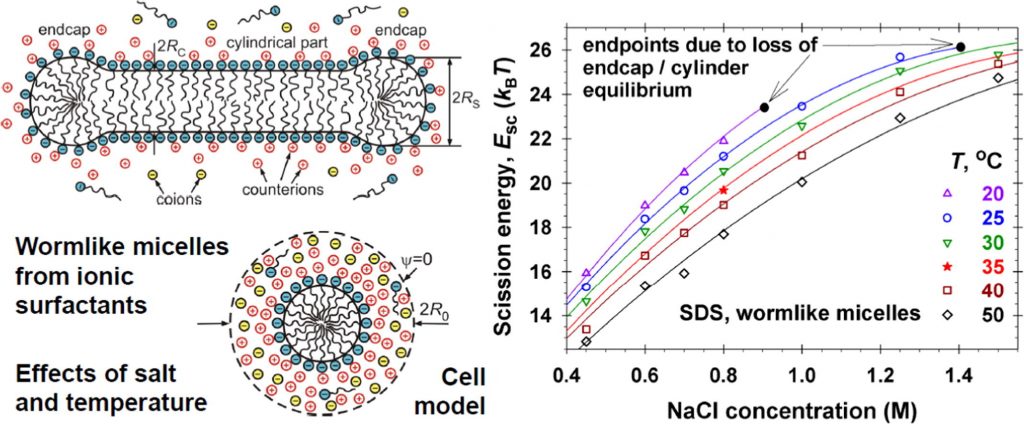
Hypotheses: The aggregation number and length of spherocylindrical (rodlike, wormlike) micelles in solutions of an ionic surfactant and salt can be predicted knowing the molecular parameters and the input concentrations of the species. This can be achieved by upgrading the quantitative molecular thermodynamic model from the previous parts of this series with an expression for the electrostatic component of micelle scission energy that is the excess free energy of the spherical endcaps with respect to the cylindrical part of the micelle. Theory: The thermodynamics of micellization is extended to the case of multicomponent system, which may contain several surfactants (both ionic and nonionic) and salts, taking into account the effect of counterion binding in the Stern layer on the micellar surface. Furthermore, the considerations are focused on a system that consists of single ionic surfactant plus salt. Findings: Excellent agreement was achieved between the theoretical model and experimental data for wormlike micelles from anionic and cationic surfactants at various concentrations of salt and temperatures. In accord with the experimental observations, at high salt concentrations, the model predicts loss of chemical equilibrium between the endcaps and cylindrical part of the wormlike micelles, which implies transition to self-assemblies of other, e.g. branched, morphology or the onset of crystallization and phase separation.

