Self-shaping of triglyceride and alkane drops: Similarities and differences
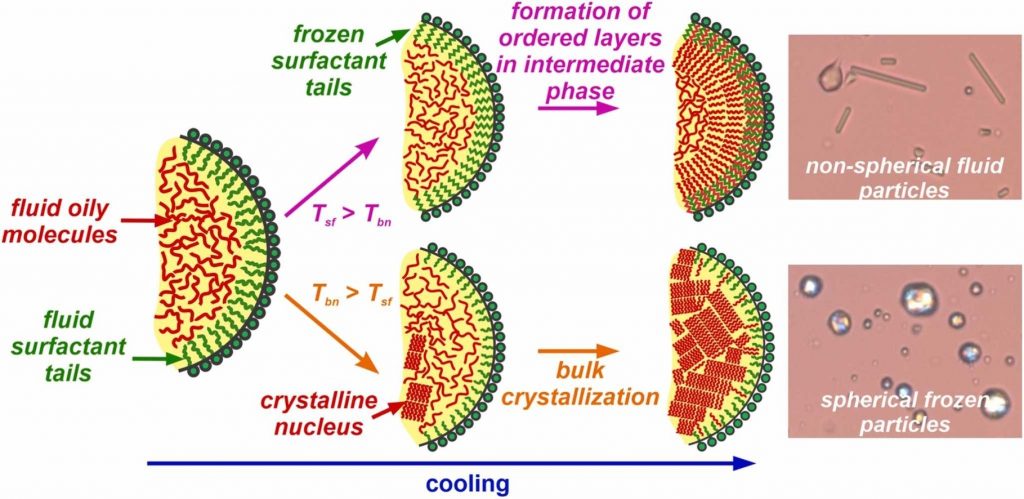
Small emulsion drops typically exhibit spherical shape at positive interfacial tension due to the energy minimization principle. However, in a series of studies (Denkov et al., Nature, 2015, 528, 392–395; Cholakova et al., Nature Phys., 2021, 17, 1050–1055) we showed that alkane droplets stabilized by appropriate saturated long-chain surfactants may spontaneously change their shape upon cooling, morphing into various polyhedra; hexagonal, tetragonal and triangular platelets; rod-like particles and even synthetic swimmers. These deformations are governed by the formation of thin plastic rotator phases adjacent to the drop surface. Although alkanes have numerous industrial applications, they cannot be used in food and pharma related products, in which most often triglyceride molecules are employed. The possibility for self-shaping of triglyceride drops has been demonstrated, but the detailed understanding of the process is currently missing. In the present study, we performed model experiments aimed to reveal the conditions under which the triglyceride emulsion drops may change their shape upon cooling. We show that most of the various non-spherical shapes known for alkanes can be reproduced with triglyceride droplets providing that the surfactant adsorption layer freezes before the nucleation of the oily molecules inside the drops. By comparing the behavior of triglyceride and alkane droplets, we draw unified picture and provide guiding principles which can be used for selection of appropriate surfactants enabling the spontaneous shape deformations upon cooling of oily drops of different chemical compositions.

