
Vesselin L. Kolev, Ph.D., Research Assoc.
Interests
- Surface Tension of Surfactant Solutions: Data Processing
- Detergency: Oil Drop Detachment
Bio
In 1999 he got the degree M.Sc. in Chemistry from the Faculty of Chemistry, Sofia University, Bulgaria, Major: “Chemical Physics and Theoretical Chemistry”. Since September 1998, he has been research associate in the Department of Chemical Engineering (DCE), Faculty of Chemistry, Sofia University, Bulgaria. During the period February 2001 – February 2004 he was PhD student. He will submit his PhD Thesis for defense in the first half of 2008. His expertise includes various theoretical and computational techniques applicable in colloid science: numerical modeling and analysis of electro-kinetic phenomena (zeta–potential), static and dynamic surface tension etc. Also, he has experience in scientific computing (grid management and creation, incl. experience in OpenMosix, MPI, OpenAFS, Scientific Linux package creation etc.), computer networks, IT security, virtualization. So far, he has published 6 research articles, cited 50 times in the scientific literature.
Publications
Most recent publications
Unit cell structure of water-filled monoolein in inverted hexagonal mesophase in the presence of incorporated tricaprylin and entrapped lysozyme
Molecular dynamics (MD) was employed by means of a specific simulation protocol to investigate the equilibrium structure at 25 °C of the hexagonal inverted (HII) mesophase composed from water, 1-monoolein (GMO), and tricaprylin, with or without entrapped lysozyme. Based on robust and fast MD simulations, the study provides a comprehensive analysis and visualization of the local structure of HII mesophase containing admixtures. The most important physical insight is the possibility to observe the strong self-recovery capacity of the GMO layer, which allows the HII mesophase tubes to reorganize and host lysozyme molecules with a size bigger than the diameter of the water channel. This is a direct message to the experimenters that the HII mesophase has the potential to host molecules larger than the diameter of the water channel. Collective character of the interlipid interactions is outlined, which is not affected by the presence of the cargo and may be the reason for the efficient GMO reorganization. Another important result is the possible explanation of the role of triacylglycerols on the low-temperature stabilization of the HII mesophase. The analysis shows that despite the low amount of tricaprylin, its molecules prevent the extreme inclination of the lipid tails and thus optimize the alignment capacity of the lipid tails layer. The study also reveals that the packing frustration does not depend on the temperature and the presence of admixtures. Hence, it might be numerically defined as a universal invariant parameter of a stable HII mesophase composed of a certain lipid.
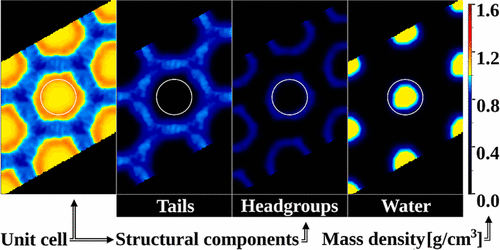
Unit cell structure of water-filled monoolein into inverted hexagonal (HII) mesophase modeled by molecular dynamics
The study investigates the unit cell structure of inverted hexagonal (HII) mesophase composed of monoolein (1-monoolein, GMO) and water using atomistic molecular dynamics methods without imposing any restraints on lipid and water molecules. Statistically meaningful and very contrast images of the radial mass density distribution, scrutinizing also the separate components water, monoolein, the polar headgroups of the lipids, the double bond, and the termini of the hydrocarbon chain (the tail), are obtained. The lipid/water interface structure is analyzed based on the obtained water density distribution, on the estimated number of hydrogen bonds per monoolein headgroup, and on the headgroup–water radial distribution functions. The headgroup mass density distribution demonstrates hexagonal shape of the monoolein/water interface that is well-defined at higher water/monoolein ratios. Water interacts with the headgroups by forming a three-layer diffusive mass density distribution, and each layer’s shape is close to hexagonal, which is an indication of long-range structural interactions. It is found that the monoolein headgroups form a constant number of hydrogen bonds leaving an excessive amount of water molecules outside the first lipid coordination sphere. Furthermore, the quantity of water at the monoolein/water interface increases steadily upon extension of the unit cell, so the interface should have a very dynamic structure. Investigation of the hydrocarbon residues reveals high compression and well-expressed structuring of the tails. The tails form a very compressed and constrained structure of defined layers across the unit cell with properties corresponding to a more densely packed nonpolar liquid (oil). Due to the hexagonal shape the 2D packing frustration is constant and does not depend on the water content. All reported structural features are based on averaging of the atomic coordinates over the time-length of the simulation trajectories. That kind of processing allows the observation of the water/GMO interface shape and its stability and mobility at a time scale close to the ones of the intermolecular interactions.
Molecular dynamics approach to water structure of HII mesophase of monoolein
The goal of the present work is to study theoretically the structure of water inside the water cylinder of the inverse hexagonal mesophase (HII) of glyceryl monooleate (monoolein, GMO), using the method of molecular dynamics. To simplify the computational model, a fixed structure of the GMOtube is maintained. The non-standard cylindrical geometry of the system required the development and application of a novel method for obtaining the starting distribution of water molecules. A predictor-corrector schema is employed for generation of the initial density of water. Molecular dynamics calculations are performed at constant volume and temperature (NVT ensemble) with 1D periodic boundary conditions applied. During the simulations the lipid structure is kept fixed, while the dynamics of water is unrestrained. Distribution of hydrogen bonds and density as well as radial distribution of water molecules across the water cylinder show the presence of water structure deep in the cylinder (about 6 Å below the GMO heads). The obtained results may help understanding the role of water structure in the processes of insertion of external molecules inside the GMO/water system. The present work has a semi-quantitative character and it should be considered as the initial stage of more comprehensive future theoretical studies.
Detachment of oil drops from solid surfaces in surfactant solutions: Molecular mechanisms at a moving contact line
Here, we present experimental data and a theoretical model for the dynamics of detachment of hexadecane drops from a solid substrate (glass plate) in aqueous solutions of anionic surfactant and salt, at various temperatures. The influence of the experimental conditions on the motion of the three-phase contact line is investigated. We found indications that water molecules can propagate by lateral diffusion in a thin layer on the surface of the solid plate. The driving force of the detachment process, viz., the imbalance of the interfacial tensions at the contact line, is engendered by the water penetration, while the line friction force compensates this imbalance and determines the stationary speed. Excellent agreement between theory and experiment is achieved. The present study specifies the parameters that can be used to quantitatively characterize the rate of drop detachment, determines the values of these parameters at various experimental conditions, and indicates tools for control of the investigated spontaneous process.
Effect of nonionic admixtures on the adsorption of ionic surfactants at fluid interfaces. 1. Sodium dodecyl sulfate and dodecanol
The main target of this study is to develop a theoretical method for determining small contents of dodecanol in samples of sodium dodecyl sulfate (SDS) by a detailed analysis of surface-tension isotherms. As a tool for our analysis, we employ the van der Waals model. Its application to data for alkanols and anionic surfactants gives an excluded area per adsorbed molecule equal to the geometrical area of the molecular cross section and adsorption energies consonant with Traube’s rule. Because the dodecanol and SDS have different excluded areas, we extended the van der Waals model for the case of a two-component adsorption layer, with account for the counterion binding in the Stern layer. General expressions for the surface free energy, two-dimensional equation of state, surface chemical potentials, adsorption isotherms, and surface dilatational elasticity are derived. The experimental surface-tension isotherms are fitted by varying only one adjustable parameter. The model was successfully tested against data for solutions of SDS with a known content of dodecanol. Knowing the parameters of the model, we computed various properties of the surfactant adsorption layer. The results show that the presence of a small amount of dodecanol leads to a considerable increase of the total adsorption and surface elasticity. Even a relatively small (0.2 mol %) fraction of dodecanol in SDS may lead to a predominant content (up to 86 mol %) of dodecanol in the mixed adsorption layer. We applied the model for determining unknown contents of dodecanol in SDS samples at different stages of purification. The addition of NaCl may lead to a significant reduction in the mole fraction of dodecanol in the adsorption layer. The developed theoretical model and computational procedure are also appropriate for a quantitative analysis and computer modeling of the adsorption from other mixed ionic-nonionic surfactant solutions, at both air-water and oil-water interfaces.

