Zhulieta N. Valkova, Ph.D.
Interests
- Emulsions and Emulsification
- Optical microscopy
- Phase Behavior of Droplets upon Cooling
Publications
Most recent publications
Rheology of dispersions containing non-spherical lipid particles
The rheological properties of disperse systems play a crucial role in the production of foods, cosmetics, and pharmaceuticals with desired characteristics. Emulsion viscosity can be increased through various methods, incl. increasing the oil volume fraction, incorporating rheological modifiers, or inducing partial coalescence between the droplets. It is well known that suspensions containing inorganic non-spherical particles often exhibit significantly higher viscosities when compared to those with spherical particles. The spontaneous drop self-shaping phenomenon in emulsions, first reported in detail by Denkov et al. (Nature, 2015, 528, 392–395), enables the formation of fluid and frozen lipid particles with regular non-spherical shapes, including platelets, rods and fibers. In this study, we utilize this approach to prepare emulsions containing non-spherical frozen particles of various shapes and investigate their rheological properties. The effects of oil volume fraction, surfactant type, initial drop size and polydispersity are investigated. The results reveal that non-flowing, gel-like samples can be prepared at ca. 11 vol% oil fraction when the emulsion contains polydisperse droplets which acquire non-spherical shapes upon cooling. For comparison, more than ca. 65 vol% oil is needed to obtain similar rheological characteristics in samples containing spherical particles. Additionally, we demonstrate that the optimal drop size for gel preparation is d32 ≈ 4–13 μm. The obtained results are explained mechanistically, and guiding principles are provided for preparing emulsions with increased viscosities using this new approach.

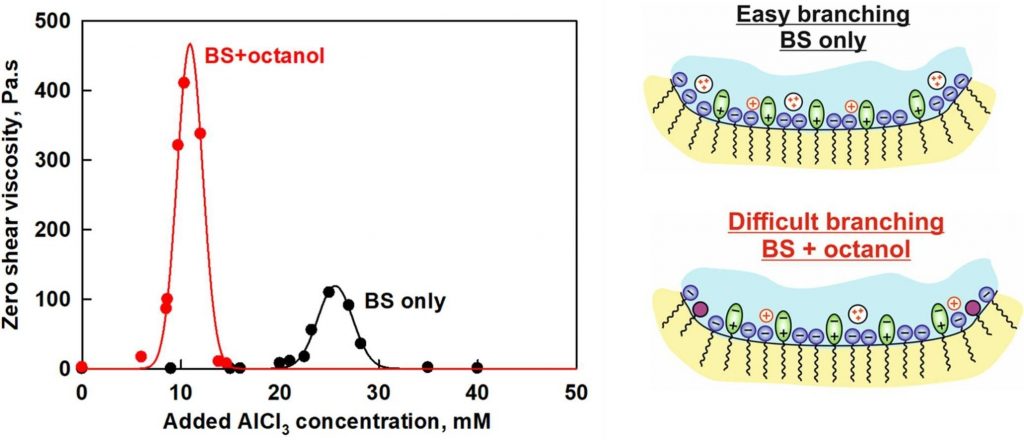
Interplay between cosurfactants and electrolytes for worm-like micelles formation
The rheological response of surfactant solutions containing a mixture of anionic and zwitterionic surfactants, in the presence of shorter-chain cationic and nonionic co-surfactants and various counterions was studied experimentally and described theoretically by developing the model that accounts for the competitive adsorption of different monovalent and divalent counterions, as well as the inclusion of co-surfactants within the micelles. This model was used to predict the salt curve dependence of systems with various salt and co-surfactant concentrations and was tested against the experimentally measured salt curves. A good agreement was found between the experimental data and the proposed theoretical model. It was demonstrated that the adsorption energies of counterions on the micellar surfaces remain unchanged with the addition of co-surfactants. However, the conditions for micelle branching are significantly affected, particularly in the presence of divalent and trivalent counterions. The presence of co-surfactants reduces the number of adsorbed divalent ions, thereby diminishing their effect on micelle branching.
Minimum surfactant concentration required for inducing self-shaping of oil droplets and competitive adsorption effects
Surfactant choice is key in starting the phenomena of artificial morphogenesis, the bottom-up growth of geometric particles from cooled emulsion droplets, as well as the bottom-up self-assembly of rechargeable microswimmer robots from similar droplets. The choice of surfactant is crucial for the formation of a plastic phase at the oil-water interface, for the kinetics, and for the onset temperature of these processes. But further details are needed to control these processes for bottom-up manufacturing and understand their molecular mechanisms. Still unknown are the minimum concentration of the surfactant necessary to induce the processes, or competing effects in a mixture of surfactants when only one is capable of inducing shapes. Here we systematically study the effect of surfactant nature and concentration on the shape-inducing behaviour of hexadecane-in-water emulsions with both cationic (CTAB) and non-ionic (Tween, Brij) surfactants over up to five orders of magnitude of concentration. The minimum effective concentration is found approximately equal to the critical micelle concentration (CMC), or the solubility limit below the Krafft point of the surfactant. However, the emulsions show low stability at the vicinity of CMC. In a mixed surfactant experiment (Tween 60 and Tween 20), where only one (Tween 60) can induce shapes we elucidate the role of competition at the interface during mixed surfactant adsorption by varying the composition. We find that a lower bound of ∼75% surface coverage of the shape-inducing surfactant with C14 or longer chain length is necessary for self-shaping to occur. The resulting technique produces a clear visual readout of otherwise difficult to investigate molecular events. These basic requirements (minimum concentration and % surface coverage to induce oil self-shaping) and the related experimental techniques are expected to guide academic and industrial scientists to formulations with complex surfactant mixtures and behaviour.
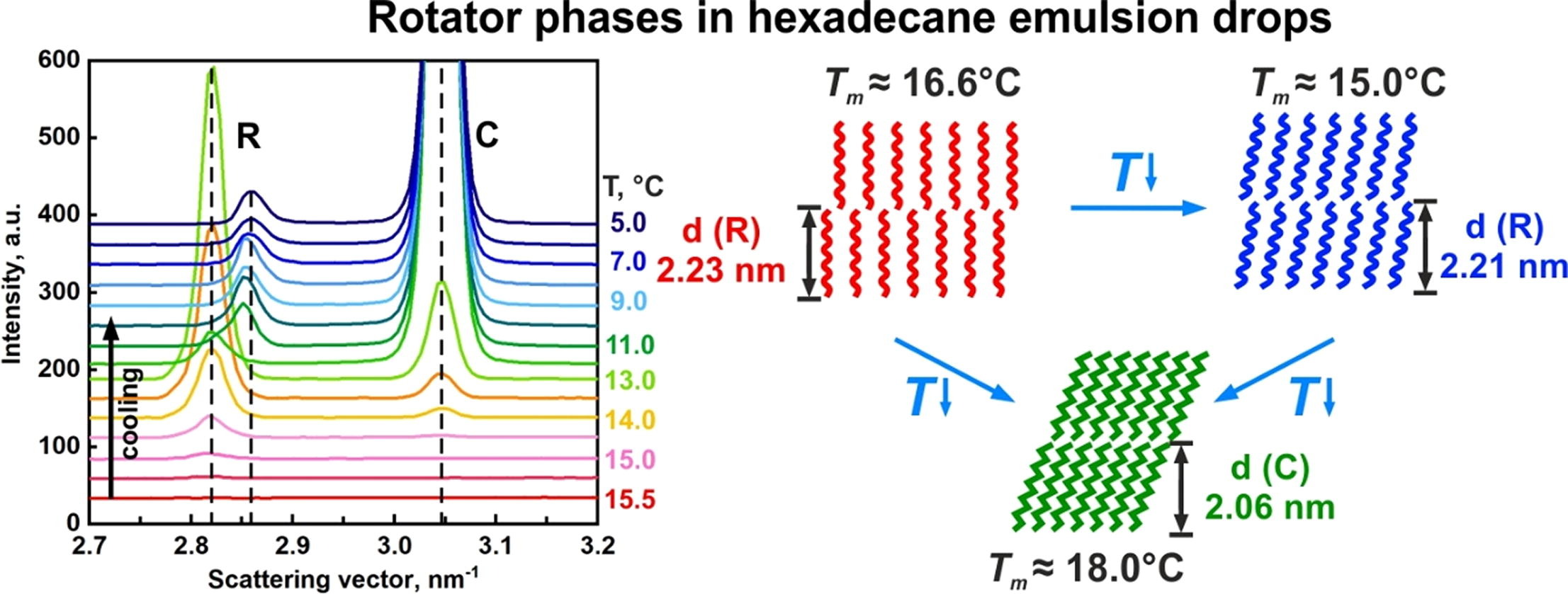
Rotator phases in hexadecane emulsion drops revealed by X-ray synchrotron techniques
Hypothesis: Micrometer sized alkane-in-water emulsion drops, stabilized by appropriate long-chain surfactants, spontaneously break symmetry upon cooling and transform consecutively into series of regular shapes (Denkov et al., Nature 2015, 528, 392). Two mechanisms were proposed to explain this phenomenon of drop “self-shaping”. One of these mechanisms assumes that thin layers of plastic rotator phase form at the drop surface around the freezing temperature of the oil. This mechanism has been supported by several indirect experimental findings but direct structural characterization has not been reported so far. Experiments: We combine small- and wide-angle X-ray scattering (SAXS/WAXS) with optical microscopy and DSC measurements of self-shaping drops in emulsions. Findings: In the emulsions exhibiting drop self-shaping, the scattering spectra reveal the formation of intermediate, metastable rotator phases in the alkane drops before their crystallization. In addition, shells of rotator phase were observed to form in hexadecane drops, stabilized by C16EO10 surfactant. This rotator phase melts at ca. 16.6 °C which is significantly lower than the melting temperature of crystalline hexadecane, 18 °C. The scattering results are in a very good agreement with the complementary optical observations and DSC measurements.