
Gergana M. Radulova, Ph.D.
Interests
- Surface rheology of protein adsorption layers – rheological models and applications
- Protein adsorption at liquid and solid interfaces
- Colloidal dispersions and micellar solutions – preparation, stability and bulk rheology
- Encapsulation of substances in inorganic shells
Publications
Most recent publications
On the rheology of linear wormlike micellar solutions
The shear rheology of linear wormlike micellar solutions (WMSs) is described by both Poisson renewal (PRM)and shuffling (SFM) models with different values of the model parameters. For low shear strains and rates ofstrains, the micellar solutions behave as a Maxwellian body with constant elasticity and viscosity. The excellentdescription of experimental data in the literature using PRM or SFM suggests that both models predict identicaldependencies of the dynamic storage and loss moduli on the frequency of oscillations. It is shown in the literature, that the PRM becomes equivalent to the SFM, when the breaking time is constant, τbr, and the characteristic reptation time, τrep, is equal to π2 τd0, where τd0 is the reptation time evaluated with respect to the average length of the chain. Three independent rheological tests (apparent viscosity vs shear rate, stress vs strain at constant shear rates, strain oscillations at low amplitudes and different frequencies) are applied to low, medium, and high zero-shear viscosity WMSs to obtain the PRM and SFM model parameters (elasticity, viscosity, relaxation, breaking, and reptation times). The known closed-form analytical expression for the Laplace image of the stress relaxation function and the respective infinite series for the complex modulus give possibility for the reported here precise systematic calculations of the storage and elastic moduli, the crossover frequency, and the elasticity for all values of ζbr = τbr/τrep ≤ 100. The predictions of the PRM length-dependent breaking-time versions are indistinguishable from those of the SFM for the obtained universal dependencies of the characteristic time, τB0, on ζbr. The applicability of the Vasquez–Cook–McKinley and the single-mode Oldroyd 8-constant models to describe the rheological behavior of WMSs is tested. The theoretical findings and conclusions are confirmed experimentally and illustrate the self-consistency of the used rheological regimes.
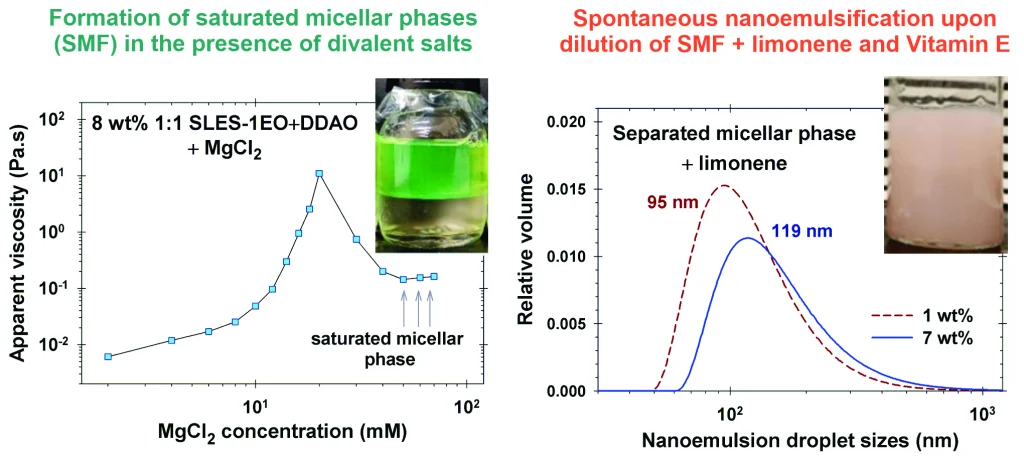
Saturated micellar networks: Phase separation and nanoemulsification capacity
Different oils can be homogeneously dispersed in the network junctions of the separated bicontinuous micellar phases. Upon dilution, these dispersions spontaneously form nanoemulsions. The possibility of a micellar sponge phase formation in the case of mixtures with three anionic and two zwitterionic surfactants in the presence of divalent and monovalent salts is studied. The best results are obtained using sodium lauryl ether sulfate with 1 ethylene oxide group (SLES-1EO) and both cocamidopropyl betaine (CAPB) or N,N-dimethyldodecylamine N-oxide (DDAO) in the presence of an appropriate small amount of MgCl2 and CaCl2. Bicontinuous micellar phases can be produced also in high-salinity NaCl solutions. The bulk properties of these phases are independent of the concentration of the initial solutions from which they are separated, and their Newtonian viscosities are in the range from 0.3 Pa·s to 0.8 Pa·s. Both 8 wt% CAPB- and DDAO-containing sponge phases engulf up to 10 wt% limonene and spontaneously form nanoemulsion upon dilution with droplet sizes of 110–120 nm. Vitamin E can be homogeneously dispersed only in CAPB-containing saturated micellar network, and upon dilution, these dispersions spontaneously form nanoemulsions with smaller droplet sizes of 66 nm for both 8 diastereomers and 2 diastereomers mixtures of vitamin E.
Growth of giant micellar aggregates: Quantitative theory vs experiments
The concentrated surfactant solutions have a wide application in industry, oil recovery, drug delivery, turbulent drag reduction, etc. The competition between the companies-producers has led to use of new kind of formulations to improve: washing action; skin and eye irritation; stability and durability; biodegradability; tolerance to hard water. Here, we present a review on the state of the art and our contributions to the molecular thermodynamic theory and experiment on the growth of giant micellar aggregates. Despite the considerable advances in theory and computer simulations, agreement with experimental data has been achieved only in isolated cases. Our predictive molecular thermodynamic approach accounts for the different contributions to the micellar scission energy in the case of nonionic, zwitterionic and ionic surfactant solutions and their mixtures. Excellent agreement was achieved between the theoretical model and experimental data for wormlike surfactant micelles at various concentrations of salt and temperatures. At high salt concentrations, the model also predicts loss of chemical equilibrium, which implies a transition to self-assemblies of other morphology or the onset of crystallization and phase separation. The results have applications for the design of new products and nanostructured materials.
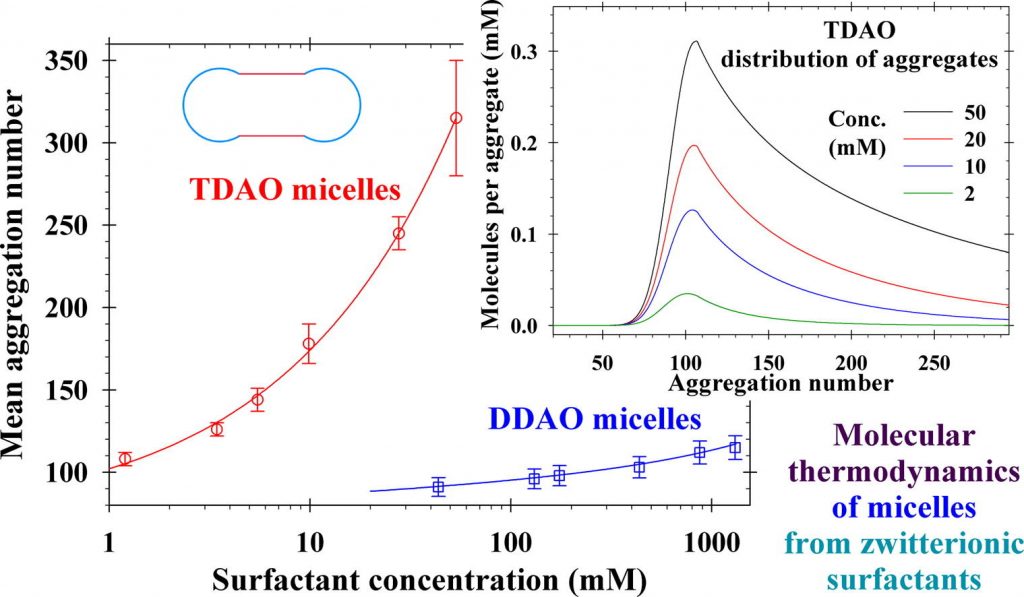
Analytical modeling of micelle growth. 5. Molecular thermodynamics of micelles from zwitterionic surfactants
Hypothesis: The critical micelle concentration, aggregation number, shape and length of spherocylindrical micelles in solutions of zwitterionic surfactants can be predicted by knowing the molecular parameters and surfactant concentrations. This can be achieved by upgrading the quantitative molecular thermodynamic model with expressions for the electrostatic interaction energy between the zwitterionic dipoles and micellar hydrophobic cores of spherical and cylindrical shapes. Theory: The correct prediction of the mean micellar aggregation numbers requires precise calculations of the free energy per molecule in the micelles. New analytical expressions for the dipole electrostatic interaction energy are derived based on the exact solutions of the electrostatic problem for a single charge close to a boundary of spherical and cylindrical dielectric media. The obtained general theory is valid for arbitrary ratios between dielectric constants, radii of spheres and cylinders, positions, and orientations of dipoles. Findings: The detailed numerical results show quantitatively the effects of the micelle curvature and dielectric properties of the continuum media on the decrease of the dipole electrostatic interaction energy. Excellent agreement was achieved between the theoretical predictions and experimental data for the critical micelle concentration, size and aggregation number of zwitterionic surfactant micelles. This study can be extended to mixed micelles of zwitterionic and ionic surfactants in the presence of salt to interpret and predict the synergistic effect on the rheology of solutions.
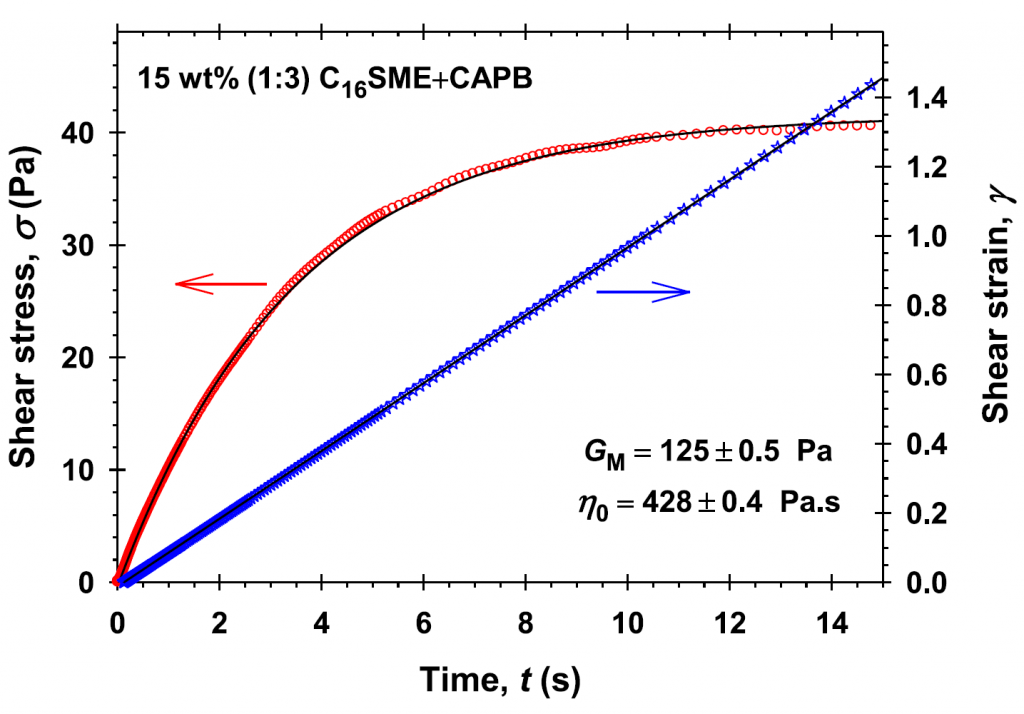
Rheology of saturated micellar networks: Wormlike micellar solutions vs. bicontinuous micellar phases
The subject of this work is to investigate the rheological behavior of mixed micellar solutions (sodium laurylethersulfate and cocamidopropyl betaine) in the presence of Mg2+ divalent counterions. With the rise of salt concentration, the viscosity of micellar solutions increases to a high maximum followed by a steep decrease because of the initial growth and entanglement of wormlike micelles and a subsequent transition to branched micelles forming of a saturated micellar network. The proposed systematic rheological measurements show considerable variations in the rheological responses of the solutions when increasing the salt concentration. Rheological behavior and data are used to distinguish the micellar phases and to study the relation to micellar structures. The wormlike micellar solutions have a typical shear thinning behavior with a well-defined zero-shear viscosity, η0, described by the Cates reptation-reaction model or the augmented Maxwell model. Our data show that the power law dependence of η0 on the surfactant concentration is stronger than that reported in the literature and it is influenced of the added electrolytes. The branched micellar structures are characterized by the lower viscosities and larger elasticities, which follow the Maxwell model up to the intermediate values of the frequency of oscillations, however peculiar deviations from the Cole-Cole plot at large frequencies are detected. The isolated bicontinuous micellar phases are Newtonian fluids with viscosity 0.4–0.7 Pa.s independent on the salt concentration up to high shear rates. The threshold salt concentration ensuring the onset of the bicontinuous micellar phase is described by a simple empirical rule. These phases are characterized by large elasticities and not negligible yield stresses. The property of the bicontinuous micellar phases to form spontaneously oil-in-water nanoemulsions could find applications in drug delivery, extraction and separation processes, pharmaceutics production, etc.

