
Borislava B. Petkova, Ph.D.
Interests
- Foam formation and stability
- Emulsions and emulsification
- Digestion and Bioavailability
- Physical chemistry of triglyceride lipolysis
- Biosurfactants: saponins, bile salts; Surface Forces
- Micelles, Solubilization and Detergency
- Kinetics of Surfactant Adsorption
- Thin Liquid Films; Chemical analysis - HPLC, GC
- Formulation of Cosmetic products
Publications
Most recent publications
Bubble size and foamability: Role of surfactants and hydrodynamic conditions
The primary objective of this review is to consolidate our current understanding of the factors controlling the foamability of surfactant solutions under hydrodynamic conditions realized in various laboratory tests. In particular, two regimes of foam generation are considered: at low surfactant concentrations where the coalescence between the bubbles plays a crucial role, and a high surfactant concentration range where the hydrodynamic conditions are much more important for the final outcome of foaming. The review discusses the role of surfactant concentration, dynamic surface coverage, and surface forces acting between film surfaces for the foam generated in the surfactant-poor regime. Additionally, the interplay between the hydrodynamic conditions and the viscosity of the formed foams in the surfactant-rich regime is also discussed.
Foamability of surfactant solutions: Interplay between adsorption and hydrodynamic conditions
The foamability of surfactant solutions depends strongly on the adsorption properties of the surfactants, their concentrations, and the foaming test used for foam generation. The aim of the current study is to analyse the subtle interplay between the hydrodynamic conditions and the surfactant adsorption layers formed during foaming. We compare the results from three foam tests, characterized with very different hydrodynamic conditions. The Bartsch test (fast-foaming method) is characterized by a very short time, of the order of milliseconds, allowed for surfactant adsorption before the newly generated bubbles collide with each other. The other two “slow-foaming” tests, the planetary mixer and the foam rise method, are very different from this viewpoint – the characteristic time for surfactant adsorption before the bubble collisions is much longer, of the order of seconds. Surfactants of two different types, nonionic and anionic, with different chain-lengths are compared. The obtained results reveal that the long-chain nonionic surfactants with 16 carbon atoms are unable to stabilize the foams in the fast-foaming test, even at very high surfactant concentrations (50 mM), due to their relatively slow adsorption. Interestingly, the same surfactants are excellent foam stabilizers in the slow-foaming tests. On the other hand, the medium-chain anionic surfactants with 12 carbon atoms are very suitable for stabilization of the gas bubbles in the fast-foaming test, even at relatively low concentrations, due to their rapid adsorption and inherent electrostatic repulsion. In slow-foaming tests, however, the anionic surfactants show lower foamability to the nonionic ones. All results from the foam tests are explained in a unified approach which accounts explicitly for the rate of surfactant adsorption and for the characteristic time-scales of the various foaming tests.
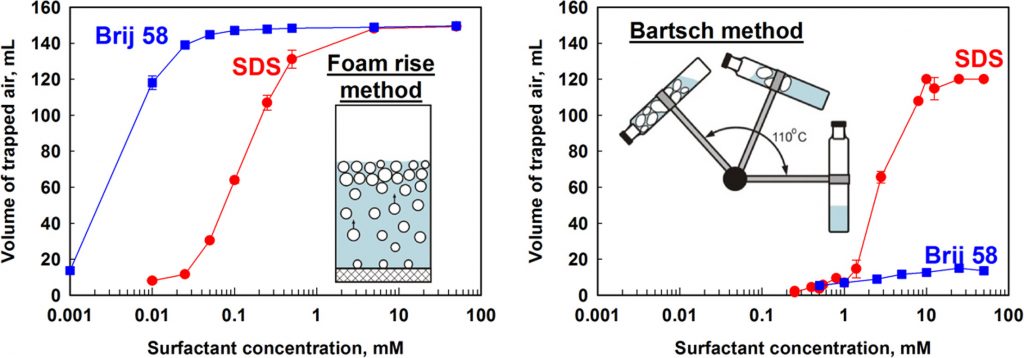
Foamability of aqueous solutions: Role of surfactant type and concentration
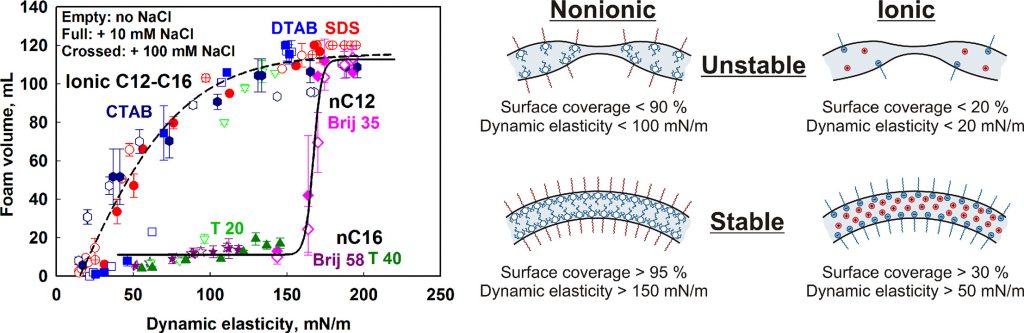
In this paper we study the main surface characteristics which control the foamability of solutions of various surfactants. Systematic series of experiments with anionic, cationic and nonionic surfactants with different head groups and chain lengths are performed in a wide concentration range, from 0.001 mM to 100 mM. The electrolyte (NaCl) concentration is also varied from 0 up to 100 mM. For all surfactants studied, three regions in the dependence of the foamability, VA, on the logarithm of surfactant concentration, lgCS, are observed. In Region 1, VA is very low and depends weakly on CS. In Region 2, VA increases steeply with CS. In Region 3, VA reaches a plateau. To analyse these results, the dynamic and equilibrium surface tensions of the foamed solutions are measured. A key new element in our interpretation of the foaming data is that we use the surface tension measurements to determine the dependence of the main surface properties (surfactant adsorption, surface coverage and surface elasticity) on the surface age of the bubbles. In this way we interpret the results from the foaming tests by considering the properties of the dynamic adsorption layers, formed during foaming. The performed analysis reveals a large qualitative difference between the nonionic and ionic surfactants with respect to their foaming profiles. The data for the nonionic and ionic surfactants merge around two master curves when plotted as a function of the surface coverage, the surface mobility factor, or the Gibbs elasticity of the dynamic adsorption layers. This difference between the ionic and nonionic surfactants is explained with the important contribution of the electrostatic repulsion between the foam film surfaces for the ionic surfactants which stabilizes the dynamic foam films even at moderate surface coverage and at relatively high ionic strength (up to 100 mM). In contrast, the films formed from solutions of nonionic surfactants are stabilized via steric repulsion which becomes sufficiently high to prevent bubble coalescence only at rather high surface coverage (> 90%) which corresponds to related high Gibbs elasticity (> 150 mN/m) and low surface mobility of the dynamic adsorption layers. Mechanistic explanations of all observed trends are provided and some important similarities and differences with the process of emulsification are outlined.
Mechanisms of cholesterol and saturated fatty acid lowering by Quillaja saponaria extract, studied by in vitro digestion model
Quillaja saponin extracts are known to reduce plasma cholesterol levels in humans. Here we study the mechanism of this effect with Quillaja Dry saponin extract (QD). In vitro model of triglyceride lipolysis is used to quantify the effect of QD on the solubilization of cholesterol and of the lipolysis products (fatty acids and monoglycerides) in the dietary mixed micelles (DMM). We found that QD extract decreases significantly both the cholesterol (from 80% to 20%) and saturated fatty acids (SFA, from 70% to 10%) solubilised in DMM. Series of dedicated experiments prove that QD may act by two mechanisms: (1) direct precipitation of cholesterol and (2) displacement of cholesterol from the DMM. Both mechanisms lead to increased cholesterol precipitation and, thus, render cholesterol bio-inaccessible. We prove also that the saponin molecules are not the active component of QD, because highly purified Quillaja extract with very similar saponin composition does not exhibit cholesterol-lowering or SFA-lowering effect. The effect of QD extract on cholesterol solubilisation is most probably caused by the high-molecular weight polyphenol molecules, present in this extract. The reduced SFA solubilisation is caused by Ca2+ ions of relatively high concentration (1.25 wt%), also present in QD extract, which precipitate the fatty acids into calcium soaps.
Effects of emulsifier charge and concentration on pancreatic lipolysis: 2. Interplay of emulsifiers and biles
As a direct continuation of the first part of our in vitro study (Vinarov et al., Langmuir2012, 28, 8127), here we investigate the effects of emulsifier type and concentration on the degree of triglyceride lipolysis, in the presence of bile salts. Three types of surfactants are tested as emulsifiers: anionic, nonionic, and cationic. For all systems, we observe three regions in the dependence degree of fat lipolysis, α, versus emulsifier-to-bile ratio, fs: α is around 0.5 in Region 1 (fs < 0.02); α passes through a maximum close to 1 in Region 2 (0.02 < fs < fTR); α is around zero in Region 3 (fs > fTR). The threshold ratio for complete inhibition of lipolysis, fTR, is around 0.4 for the nonionic, 1.5 for the cationic, and 7.5 for the anionic surfactants. Measurements of interfacial tensions and optical observations revealed the following: In Region 1, the emulsifier molecules are solubilized in the bile micelles, and the adsorption layer is dominated by bile molecules. In Region 2, mixed surfactant-bile micelles are formed, with high solubilization capacity for the products of triglyceride lipolysis; rapid solubilization of these products leads to complete lipolysis. In Region 3, the emulsifier molecules prevail in the adsorption layer and completely block the lipolysis.

