
Gergana S. Tsenova (Georgieva), Ph.D.
Interests
- Surface Forces in Colloidal Dispersions
- Micellization and Self-Assembly
- Rheology
- Phase behavior of carboxylate solutions
- Light-scattering and electrophoretic methods
- Microbiological and antibacterial properties
Bio
Gergana S. Tsenova (Georgieva) received M.Sc. in Colloid Chemistry (2012), Ph.D. in Physical/Theoretical Chemistry (2018) in the Faculty of Chemistry and Pharmacy, Sofia University, Bulgaria. She has been a STSM researcher in the Research Center Paul Pascal, CNRS, Bordeaux, France (2013). Her research interests include: Surface Forces in Colloidal Dispersions; Micellization and Self-Assembly; Rheology; Phase behavior of carboxylate solutions; Light-scattering and electrophoretic methods; Microbiological and antibacterial properties. So far, she has published 7 research articles, cited over 200 times in the scientific literature (h-index = 6). She participated in more than 10 projects with international companies (Unilever, SCJ, Lonza, Sasol etc.).
Publications
Most recent publications
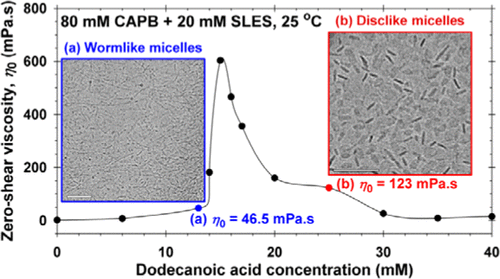
Viscosity peak due to shape transition from wormlike to disklike micelles: Effect of dodecanoic acid
Here, we have investigated the synergistic growth of long wormlike micelles and their transformation into disklike micelles, which occurs in three-component solutions composed of sodium lauryl ether sulfate (SLES; anionic), cocamidopropyl betaine (CAPB; zwitterionic), and dodecanoic acid (HC12; nonionic). The solution rheology is characterized in terms of zero-shear viscosities and characteristic times for micellar breaking and reptation. Furthermore, the microstructure evolution, leading to the observed rheological behavior, is revealed by cryo-transmission electron microscopy (TEM) micrographs. In all cases, the CAPB-to-SLES ratio is fixed, whereas the fatty acid concentration is varied. At a certain HC12 concentration, the solution viscosity passes through a maximum. The cryo-TEM imaging indicates that wormlike micelles appear before the peak, grow further up to the peak, and finally transform into disklike aggregates (a very rare micellar structure) after the peak. The transformation of worms into disks leads to a drop in viscosity because the length-to-thickness aspect ratio of the disks is significantly lower than that of the worms. In this article, we elucidate the structure-rheology relations in micellar solutions that might be applied for the design of personal-care and household formulations.
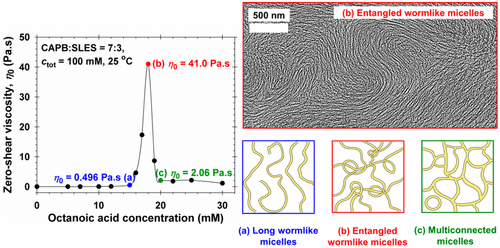
Synergistic growth of giant wormlike micelles in ternary mixed surfactant solutions: Effect of octanoic acid
The synergistic growth of giant wormlike micelles in ternary mixed solutions composed of an anionic surfactant (sodium laurylethersulfate, SLES), a zwitterionic surfactant (cocamidopropyl betaine, CAPB), and octanoic acid (HC8) is studied. Rheological data and their analysis in terms of Cole-Cole plots and micellar characteristic times are presented, and the micellar structures behind the observed rheological behavior are revealed by cryo-TEM micrographs. The surfactant composition is fixed near the maximal micelle size of the binary SLES + CAPB system, whereas the concentration of HC8 is varied. At a given HC8 concentration, the viscosity of the ternary micellar solutions exhibits a very high and sharp peak. Polarized-light optical microscopy indicates that all investigated solutions are isotropic rather than liquid-crystalline. The cryo-TEM imaging shows complex phase behavior: wormlike micelles to the left of the peak, giant entangled wormlike micelles at the peak, and long wormlike micelles coexisting with multiconnected micellar aggregates to the right of the peak. The formation of multiconnected micelles leads to a drop in viscosity at the higher concentrations. The results contribute to a better understanding of the structure-rheology relations in micellar surfactant solutions and could be useful for controlling the properties of formulations in personal-care and house-hold detergency.
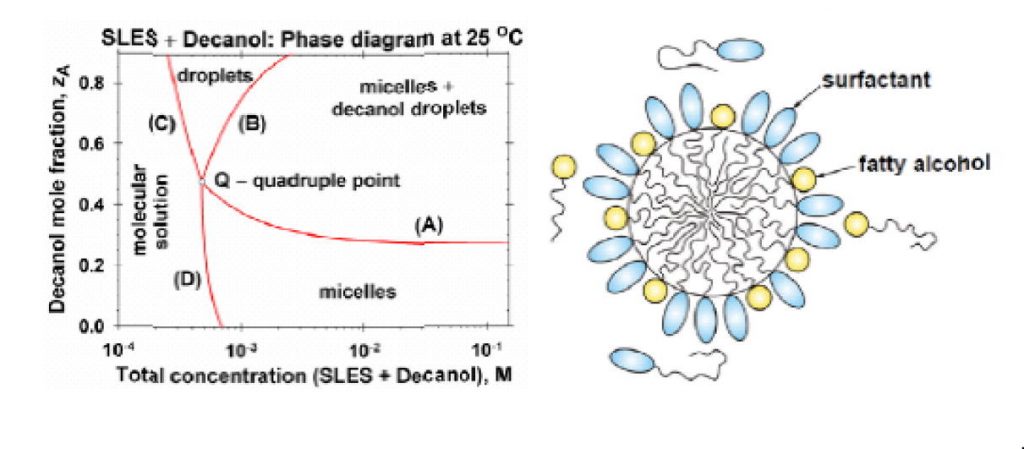
Solubility limits and phase diagrams for fatty alcohols in anionic (SLES) and zwitterionic (CAPB) micellar surfactant solutions
By analysis of experimental data, a quantitative theoretical interpretation of the solubility limit of medium- and long-chain fatty alcohols in micellar solutions of water-soluble surfactants is presented. A general picture of the phase behavior of the investigated systems is given in the form of phase diagrams. The limited solubility of the fatty alcohols in the micelles of conventional surfactants is explained with the precipitation of their monomers in the bulk, rather than with micelle phase separation. The long chain fatty alcohols (with n= 14, 16 and 18 carbon atoms) exhibit an ideal mixing in the micelles of the anionic surfactant sodium laurylethersulfate (SLES) and the zwitterionic surfactant cocamidopropyl betaine (CAPB) at temperatures of 25, 30, 35 and 40. °C. Deviations from ideality are observed for the alcohols of shorter chain (n= 10 and 12), which can be explained by a mismatch with the longer chains of the surfactant molecules. Using the determined thermodynamic parameters of the systems, their phase diagrams are constructed. Such a diagram consists of four domains, viz. mixed micelles; coexistent micelles and precipitate (dispersed crystallites or droplets); precipitate without micelles, and molecular solution. The four boundary lines intersect in a quadruple point, Q. For ionic surfactants (like SLES), a detailed theory for calculating the boundary lines of the phase diagrams is developed and verified against data for the positions of the kinks in surface tension isotherms. The theory takes into account the electrostatic interactions in the micellar solutions and the effect of counterion binding. The results can be useful for a quantitative interpretation and prediction of the phase behavior of mixed solutions of two (or more) surfactants, one of them being water soluble and forming micelles, whereas the other one has a limited water solubility, but readily forms mixed micelles with the former surfactant.
Disclike vs. cylindrical micelles: Generalized model of micelle growth and data interpretation
Here, we present a detailed theoretical model describing the growth of disclike surfactant micelles. The model is tested against light-scattering data for micellar solutions from mixed conventional surfactants and from fluorinated surfactants. Theoretical expressions are derived for the concentration dependencies of the number and mass average aggregation numbers. Central role in the theory is played by the difference between the chemical potentials of a surfactant molecule in cylindrical and discoidal micelles. This difference, scaled with the thermal energy kT, is denoted p. For p. 0 disclike micelles are formed, but their growth is limited due to the rise of their positive peripheral energy. Because of that, disclike micelles can be observed in a relatively narrow interval, 0. <. p<. 0.1, and in a limited concentration range. Three sets of light-scattering data for different surfactants were processed. It is remarkable that in all cases the best fit gives small positive values of p, in agreement with the theoretical predictions. The model predicts that a strong increase in the viscosity of a surfactant solution should happen upon the transformation of disclike micelles into cylindrical ones at small variations in p. The model can be used for analyzing the shape and size of micelles in various surfactant solutions. The fact that typical disclike micelles form only in the special case 0. <. p<. 0.1 shows why such micelles represent a relatively rare form of stable surfactant self-assembly.
Extension of the ladder model of self-assembly from cylindrical to disclike surfactant micelles
The ladder model of growth of cylindrical micelles gives expressions for the micellar size distribution and for the mean aggregation number, which are in good agreement with the experiment. Here, we consider this model and its extension to the case of disclike micelles. In analogy with the modeling of elongated micelles as sphero-cylinders, the disclike micelles can be modeled as toro-discs. Upon micelle growth, the hemispherical caps of a cylindrical aggregate remain unchanged, whereas the semitoroidal periphery of a disclike micelle expands. This effect can be taken into account in the expression for the size distribution of the disclike micelles, which predicts the dependence of the micelle mean aggregation number on the surfactant concentration. It turns out that disclike micelles could form in a limited range of surfactant concentrations, and that their mean aggregation number cannot exceed a certain maximal value. Large disclike micelles can exist only near the border with the domain of cylindrical micelles. Then, small variations in the experimental conditions could induce a transformation of the disclike micelles into cylindrical ones.

