Zlatina G. Mitrinova, Ph.D.
Interests
- Foam formation, stability and rheology
- Surfactant solution phase behavior and rheology
Bio
Zlatina Mitrinova received M.Sc. in Colloidal systems in contemporary science and technology (2010), Ph.D. in Physical chemistry-microkinetics (2015), in the Faculty of Chemistry and Pharmacy, Sofia University, Bulgaria. She is PostDoc in the Department since January 2015. She has been researcher R2 in a projects with public finances. Her research interests include: foam formation and stability ; rheology of foams ; foam stability in the presence of antifoams; surfactant solutions aggregation and rheology. So far, she has published 8 research articles, cited over 184 times in the scientific literature (h-index =5). She has been participating in projects with international companies (Unilever, Wacker, Lubrizol, Altana, BYK Chemie).
Publications
Most recent publications
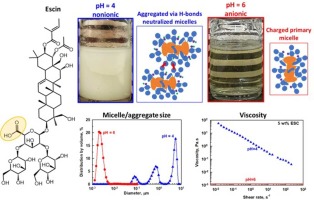
Escin solutions: Effects of pH and electrolytes on their behavior
Escin is a triterpenoid saponin with one carboxyl group which is non-ionized at pH 4.7. The major aim of the current study is to determine how the electrolyte concentration affects the properties of concentrated escin solutions (5 wt% and 10 wt%) at pHs of 4, 6, and 8. Ionized escin molecules at pH >4.7 form charged micelles that repel one another when there is no added electrolyte and solutions remain clear and stable for more than a month. Lowering the pH to 4 leads to formation of uncharged micelles. These micelles attract each other and form inter-micellar hydrogen bonds, which enable formation of micrometer aggregates that cause turbidity and phase separation. The addition of background electrolytes to the solutions at pHs of 6 and 8 screens the electrostatic repulsion between micelles, causing partial aggregation of the micelles and gelation of solutions. As the salt concentration increases, the viscosity of the escin solution also increases, reaching a maximum—similar to the behavior observed with conventional surfactants. However, the mechanism behind this viscosity maximum is different. In solutions of conventional surfactants, the maximum is due to the formation of worm-like micelles, whereas the maximum for escin solutions is due to formation of a network of escin aggregates that imparts yield stress and elasticity to the solution. These dispersions remain stable for at least one month at room temperature and can be used as cosmetic and detergent formulations.
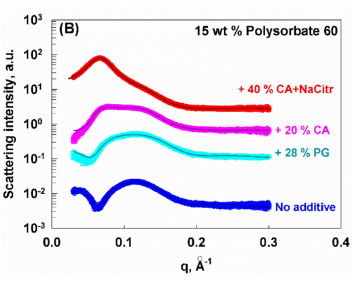
Role of food additives on properties of Polysorbate 60 solutions
Polysorbates are hydrophilic, nonionic surfactants widely used in food, cosmetic, and pharmaceutical products. Often, these emulsifiers are used in combination with different preservatives, and it is essential to conduct an in-depth study of the influence of such additives on the properties of Polysorbate 60 solutions. In the current study, we investigated the effect of various food additives: citric acid, sodium benzoate, potassium sorbate, a mixture of citric acid and sodium citrate, and propylene glycol on the stability and micellar properties of Polysorbate 60 by using different experimental methods such as GC, DSC, SAXS, DLS, optical observations in polarised light, and NMR. When stored at room temperature without additives, solutions of Polysorbate 60 slowly undergo phase separation over time. Our results show that citric acid, a mixture of citric acid and sodium citrate, and propylene glycol increase the rate of this phase separation. In contrast, sodium benzoate and potassium sorbate are incorporated into the mixed micelles. They localize within the palisade layer, a region of the micelle where the surfactant tails begin, effectively preventing the phase separation. As a result, Polysorbate 60 solutions with these specific additives remain transparent and stable for over a year
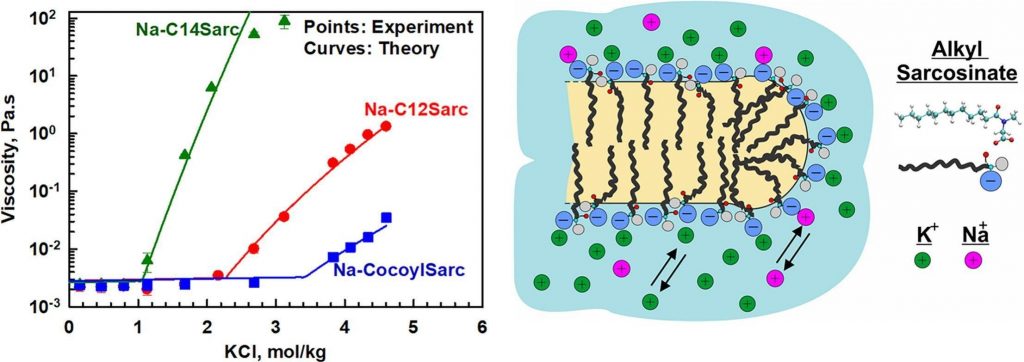
Role of electrolytes and co-surfactants on the rheological properties of sodium N-acyl sarcosinate solutions
Alkyl sarcosinates are amino acid-based anionic surfactants commonly used as primary surfactants in sulfate-free personal care products. The major aim of this study is to identify the key factors influencing the rheological behaviour of sodium sarcosinate solutions and their mixtures with nonionic, zwitterionic, and cationic co-surfactants. To achieve this, we examined the effects of salt type and concentration for alkyl sarosinates with different chain lengths (dodecyl, tetradecyl, and cocoyl) across concentration range of 2–20 wt%. Experimental results reveal two distinct regions in the salt curve for the three studied sarcosinates. At low electrolyte concentrations, viscosity remains constant until reaching the critical electrolyte concentration, C1, beyond which viscosity increases logarithmically with salt concentration. Further electrolyte addition leads to phase separated solutions at the critical precipitation concentration, CTR. Both C1 and CTR decrease as the hydrocarbon chain length increases from dodecyl to tetradecyl. However, the presence of shorter chain molecules in cocoyl sarocisinate significantly increases both C1 and CTR due to the formation of spherical micelles. A theoretical expression for predicting viscosity dependence on salt concentration is derived and successfully applied to describe the experimental data. The adsorption energy of sodium and potassium to alkyl sacrosinate micelle surfaces is found to be much smaller than that to sodium lauroyl ether sulfate surfactants (1 vs. 3 kBT for Na+ and 0.8 vs. 3.8 kBT for K+). No significant effect of amphoteric co-surfactants, including cocoamidopropyl betaine, sulfobetaine, or decylamine oxide, is observed. NMR analysis confirms that cocoamidopropyl betaine and sodium dodecyl sarcosinate form mixed micelles that are structurally similar to sarcosinate micelles, as carboxyl groups remain exposed on the micelle surfaces in both cases. When using amine oxide and sulfobetaine, the increase in viscosity is attributed to the elongation of mixed micelles, though steric hindrance from side methyl groups limits their growth. The practical significance of this study lies in the finding that longer-chain alkyl sarcosinates (such as tetradecyl, as investigated here) can attain significantly higher viscosities at lower salt concentrations compared to shorter-chain analogs or surfactant mixtures. The scientific significance stems from the development of a theoretical model capable of predicting the viscosity of alkyl sarcosinate solutions across various surfactant and salt concentrations.
Interplay between cosurfactants and electrolytes for worm-like micelles formation
The rheological response of surfactant solutions containing a mixture of anionic and zwitterionic surfactants, in the presence of shorter-chain cationic and nonionic co-surfactants and various counterions was studied experimentally and described theoretically by developing the model that accounts for the competitive adsorption of different monovalent and divalent counterions, as well as the inclusion of co-surfactants within the micelles. This model was used to predict the salt curve dependence of systems with various salt and co-surfactant concentrations and was tested against the experimentally measured salt curves. A good agreement was found between the experimental data and the proposed theoretical model. It was demonstrated that the adsorption energies of counterions on the micellar surfaces remain unchanged with the addition of co-surfactants. However, the conditions for micelle branching are significantly affected, particularly in the presence of divalent and trivalent counterions. The presence of co-surfactants reduces the number of adsorbed divalent ions, thereby diminishing their effect on micelle branching.
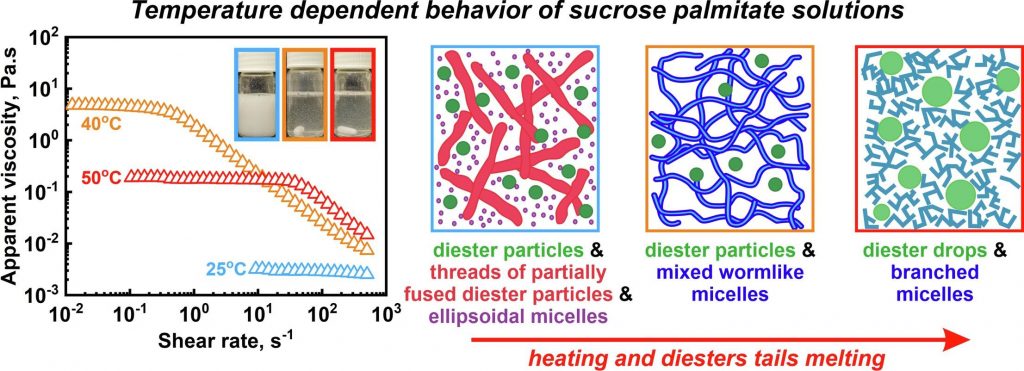
Temperature response of sucrose palmitate solutions: Role of ratio between monoesters and diesters
Hypothesis: Aqueous solutions of long-chain water-soluble sucrose ester surfactants exhibit non-trivial response to temperature variations, revealing a peak in viscosity around 40–50 ◦C. While previous investigations have explored the structures within sucrose stearate systems at various constant temperatures, a comprehensive understanding of the entire temperature dependence and the underlying molecular factors, contributing to this phenomenon is currently missing. Experiments: Temperature dependent properties and supramolecular structures formed in aqueous solutions of commercial sucrose palmitate were examined using SAXS/WAXS, DSC, optical microscopy, rheological measurements, NMR, and cryo-TEM. Findings: The underlying mechanism governing this unusual behavior is revealed and is shown to relate to the mono- to di-esters ratio in the solutions. Solutions primarily containing sucrose monoesters (monoesters molecules ≳ 98% of all surfactant molecules) exhibit behavior typical of nonionic surfactants, with minimal changes with temperature. In contrast, the coexistence of mono- and di-esters results in the formation of discrete monodisperse diester particles and a network of partially fused diester particles at low temperature. As the temperature approaches the diesters’ melting point, wormlike mixed micelles form, causing a viscosity peak. The height of this peak increases significantly with the diester concentration. Further temperature increase leads to fluidization of surfactant tails and formation of branched micelles, while excess diester molecules phase separate into distinct droplets.