Dilek F. Gazolu-Rusanova, Ph.D.
Interests
- Contact angle, wetting
- Surfactants, micellar solutions, adsorption
- Emulsions, emulsification, micro- and nanoemulsions
Bio
Publications
Most recent publications
Role of food additives on properties of Polysorbate 60 solutions
Polysorbates are hydrophilic, nonionic surfactants widely used in food, cosmetic, and pharmaceutical products. Often, these emulsifiers are used in combination with different preservatives, and it is essential to conduct an in-depth study of the influence of such additives on the properties of Polysorbate 60 solutions. In the current study, we investigated the effect of various food additives: citric acid, sodium benzoate, potassium sorbate, a mixture of citric acid and sodium citrate, and propylene glycol on the stability and micellar properties of Polysorbate 60 by using different experimental methods such as GC, DSC, SAXS, DLS, optical observations in polarised light, and NMR. When stored at room temperature without additives, solutions of Polysorbate 60 slowly undergo phase separation over time. Our results show that citric acid, a mixture of citric acid and sodium citrate, and propylene glycol increase the rate of this phase separation. In contrast, sodium benzoate and potassium sorbate are incorporated into the mixed micelles. They localize within the palisade layer, a region of the micelle where the surfactant tails begin, effectively preventing the phase separation. As a result, Polysorbate 60 solutions with these specific additives remain transparent and stable for over a year
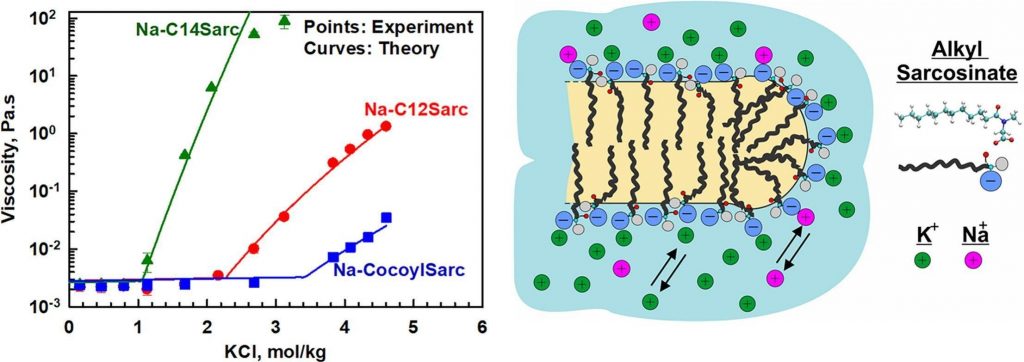
Role of electrolytes and co-surfactants on the rheological properties of sodium N-acyl sarcosinate solutions
Alkyl sarcosinates are amino acid-based anionic surfactants commonly used as primary surfactants in sulfate-free personal care products. The major aim of this study is to identify the key factors influencing the rheological behaviour of sodium sarcosinate solutions and their mixtures with nonionic, zwitterionic, and cationic co-surfactants. To achieve this, we examined the effects of salt type and concentration for alkyl sarosinates with different chain lengths (dodecyl, tetradecyl, and cocoyl) across concentration range of 2–20 wt%. Experimental results reveal two distinct regions in the salt curve for the three studied sarcosinates. At low electrolyte concentrations, viscosity remains constant until reaching the critical electrolyte concentration, C1, beyond which viscosity increases logarithmically with salt concentration. Further electrolyte addition leads to phase separated solutions at the critical precipitation concentration, CTR. Both C1 and CTR decrease as the hydrocarbon chain length increases from dodecyl to tetradecyl. However, the presence of shorter chain molecules in cocoyl sarocisinate significantly increases both C1 and CTR due to the formation of spherical micelles. A theoretical expression for predicting viscosity dependence on salt concentration is derived and successfully applied to describe the experimental data. The adsorption energy of sodium and potassium to alkyl sacrosinate micelle surfaces is found to be much smaller than that to sodium lauroyl ether sulfate surfactants (1 vs. 3 kBT for Na+ and 0.8 vs. 3.8 kBT for K+). No significant effect of amphoteric co-surfactants, including cocoamidopropyl betaine, sulfobetaine, or decylamine oxide, is observed. NMR analysis confirms that cocoamidopropyl betaine and sodium dodecyl sarcosinate form mixed micelles that are structurally similar to sarcosinate micelles, as carboxyl groups remain exposed on the micelle surfaces in both cases. When using amine oxide and sulfobetaine, the increase in viscosity is attributed to the elongation of mixed micelles, though steric hindrance from side methyl groups limits their growth. The practical significance of this study lies in the finding that longer-chain alkyl sarcosinates (such as tetradecyl, as investigated here) can attain significantly higher viscosities at lower salt concentrations compared to shorter-chain analogs or surfactant mixtures. The scientific significance stems from the development of a theoretical model capable of predicting the viscosity of alkyl sarcosinate solutions across various surfactant and salt concentrations.
Process for preparing transparent emulsions
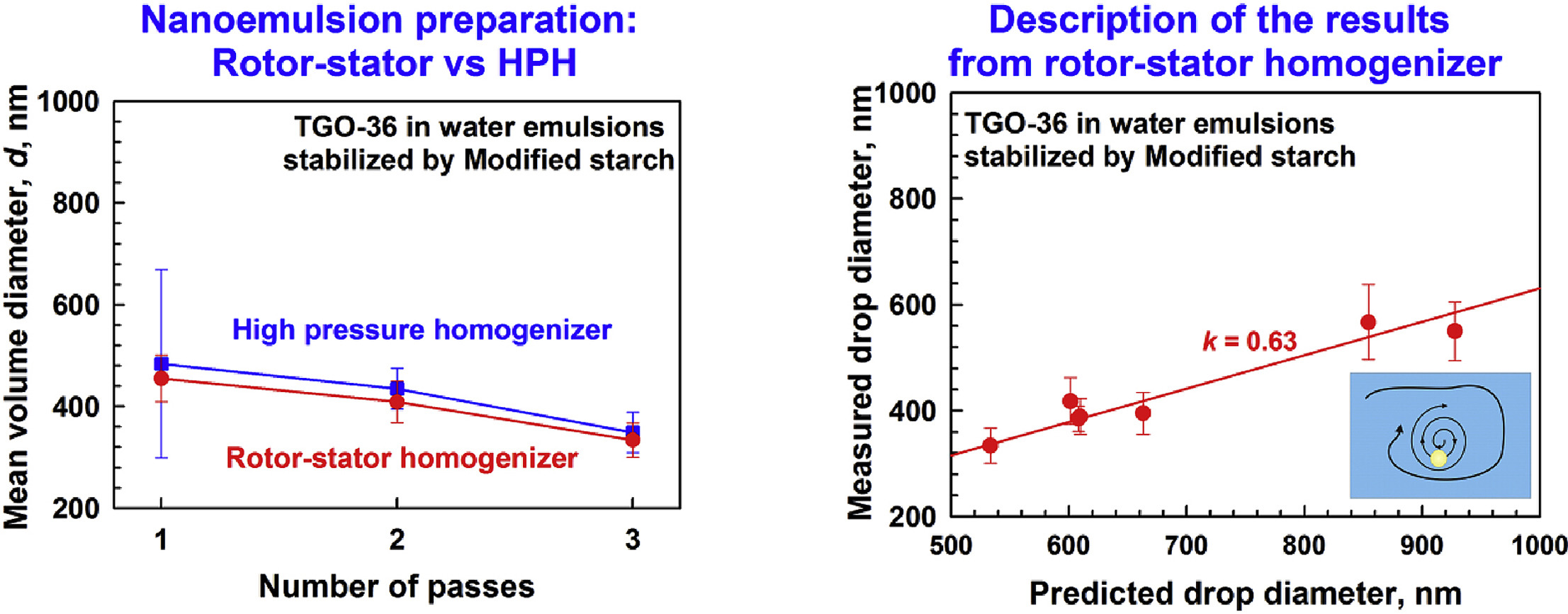
Food grade nanoemulsions preparation by rotor-stator homogenization
High-pressure homogenizers, typically used for producing nanoemulsions at the industrial scale, are energy and maintenance intensive, and limited to produce only dilute, low viscosity nanoemulsions. We propose an alternative approach to produce dilute to concentrated food-grade nanoemulsions with droplet size ranging between 100 and 500 nm using rotor-stator homogenization. Gum Arabic (GA) or modified starch (MS) was used as both viscosity modifier and emulsion stabilizer. GA and MS have relatively low surface activity compared to the common low-molecular-mass surfactants used typically for nanoemulsion preparation. The main differences between GA and MS are the lower viscosity of the GA solutions, compared to MS solutions, and the faster adsorption of MS, as compared to GA. The obtained results show that stable nanoemulsions are formed by rotor-stator homogenization when the rapidly adsorbing MS is used as emulsifier. Much larger drops are formed during emulsification with GA, which is due to significant drop-drop coalescence in the respective emulsions. The experimental results for the nanoemulsions prepared with MS are well-described by the theoretical expression for emulsification in turbulent viscous regime, after proper account for the effects of temperature and drop-drop interactions in the sheared emulsions.
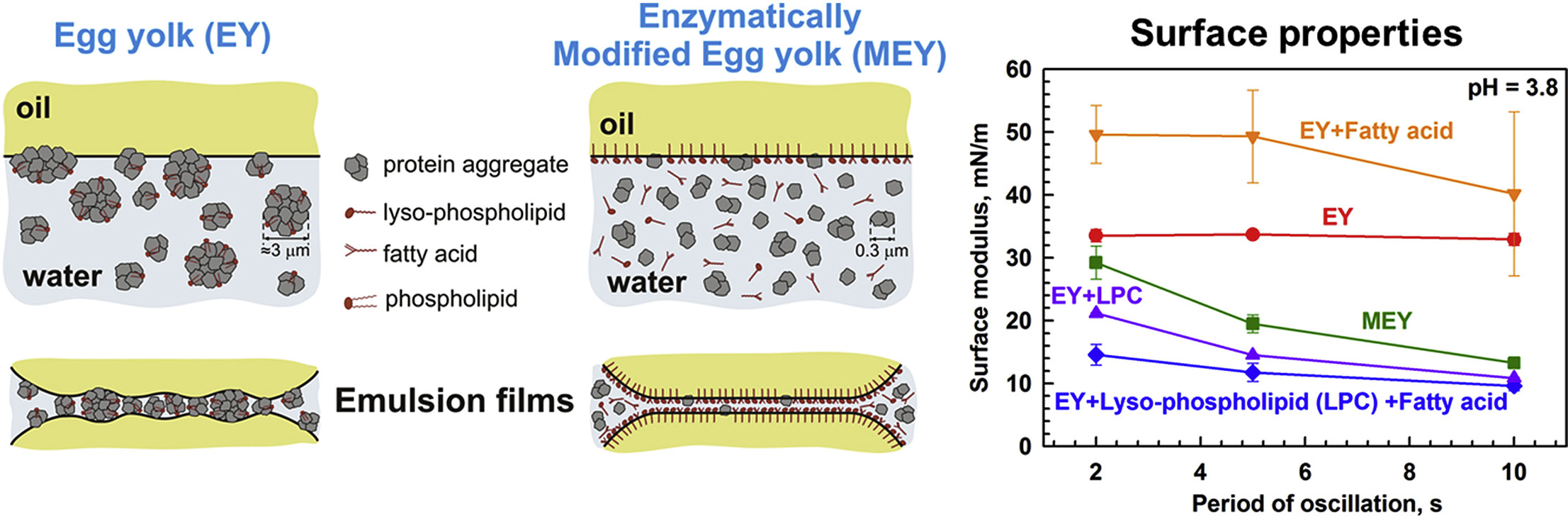
Role of lysophospholipids on the interfacial and liquid film properties of enzymatically modified egg yolk solutions
This study aims to clarify and explain the similarities and differences in the behavior of adsorption layers of native egg yolk (EY) and enzymatically modified egg yolk (MEY) at a soybean oil-water interface. For this purpose, the interfacial tension and the surface dilatational modulus of EY and MEY solutions are measured and compared. The interactions between two adsorption layers, formed from these solutions on an oil-water or air-water interface, are also studied by optical observations of thin foam and emulsion films, formed in a capillary cell. The chemical composition, the electrophoretic mobility of the molecular aggregates, and the rheological properties of the egg yolk solutions are also characterized. Adsorption layers formed from MEY solutions display a faster rate of adsorption, lower dilatational surface moduli and higher equilibrium surface tension. The enzymatic modification of egg yolk also leads to formation of much thinner foam and emulsion films and to faster film thinning. The observed differences between EY and MEY are explained by assuming that the interfacial properties of MEY are governed mostly by the lysophospholipids and oleic acid, which appear as reaction products of the enzymatic modification of EY. The latter assumption is unambiguously proven by chemical analysis of the MEY solutions and by deliberate addition of lysophospholipids and oleic acid to the non-modified EY solutions. Even at relatively low concentrations, the lysophospholipids and oleic acid change the interfacial and film properties of the EY solutions, making them very similar to those of the enzymatically modified egg yolk.